Key Points
Cytomegalovirus after bone marrow transplantation remains associated with lower survival but not prevention of leukemia relapse.
Abstract
Single-center studies have reported an association between early (before day 100) cytomegalovirus (CMV) reactivation and decreased incidence of relapse for acute myeloid leukemia (AML) following allogeneic hematopoietic cell transplantation. To substantiate these preliminary findings, the Center for International Blood and Marrow Transplant Research (CIBMTR) Database was interrogated to analyze the impact of CMV reactivation on hematologic disease relapse in the current era. Data from 9469 patients transplanted with bone marrow or peripheral blood between 2003 and 2010 were analyzed according to 4 disease categories: AML (n = 5310); acute lymphoblastic leukemia (ALL, n = 1883); chronic myeloid leukemia (CML, n = 1079); and myelodysplastic syndrome (MDS, n = 1197). Median time to initial CMV reactivation was 41 days (range, 1-362 days). CMV reactivation had no preventive effect on hematologic disease relapse irrespective of diagnosis. Moreover, CMV reactivation was associated with higher nonrelapse mortality [relative risk [RR] among disease categories ranged from 1.61 to 1.95 and P values from .0002 to <.0001; 95% confidence interval [CI], 1.14-2.61). As a result, CMV reactivation was associated with lower overall survival for AML (RR = 1.27; 95% CI, 1.17-1.38; P <.0001), ALL (RR = 1.46; 95% CI, 1.25-1.71; P <.0001), CML (RR = 1.49; 95% CI, 1.19-1.88; P = .0005), and MDS (RR = 1.31; 95% CI, 1.09-1.57; P = .003). In conclusion, CMV reactivation continues to remain a risk factor for poor posttransplant outcomes and does not seem to confer protection against hematologic disease relapse.
Introduction
Recipients of allogeneic hematopoietic transplant (HCT) who have positive cytomegalovirus (CMV) serology are at increased risk for CMV reactivation and early and late nonrelapse mortality (NRM).1 Current viral surveillance through polymerase chain reaction (PCR) and preemptive antiviral therapy for CMV reactivation have reduced the risk of death from CMV disease to <10%.2-6 However, the need for novel therapies remain, as current antiviral therapy is associated with significant side effects including renal insufficiency and bone marrow suppression, and certain viral infections in HCT patients, like CMV pneumonia,7 are associated with high mortality rates.
Some single-center studies have noted an unexpected association of positive CMV serology or early (before 100 days after HCT [D100]) CMV reactivation with decreased incidence of hematologic disease relapse following allogeneic HCT.8-13 Although initially described in preventing acute myelogenous leukemia (AML) relapse, this putative protective effect of CMV reactivation has also been observed against chronic myeloid leukemia (CML) relapse.11 Furthermore, lower hematologic disease relapse has translated into improvements in overall survival (OS) in some studies.8,9,12 In contrast, some pediatric studies do not suggest a protective effect of CMV reactivation on hematologic disease relapse,14 and even others suggest an actual increased risk in hematologic disease relapse.15 Some adult studies have also found no correlation between CMV serology and relapse.16,17 Moreover, a recent analysis from the European Society for Blood and Marrow Transplantation (EBMT) showed a higher risk of leukemia relapse and poorer OS associated with positive CMV serology in allogeneic HCT recipients.18
Given these conflicting data, the Center for International Blood and Marrow Transplant Research (CIBMTR) database was queried to define further the impact of CMV serostatus and reactivation on hematologic disease relapse, OS, and NRM following allogeneic HCT.
Materials and methods
Data source
The CIBMTR is a working group of >500 transplant centers worldwide that collects detailed information on autologous and allogeneic HCT patients, diagnoses, and outcomes. Data are collected at the Medical College of Wisconsin or through the National Marrow Donor Program. Computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. The CIBMTR collects both Transplant Essential Data and Comprehensive Report Form data before transplantation at 100 days (D100) and at 6 months (D180) after HCT and annually thereafter. All patients whose data were included in this study provided institutional review board-approved consent to participate in the CIBMTR Research Database and have their data included in observational research studies. The institutional review board of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Data collection and criteria for selection
All patients reporting to the CIBMTR who received first allogeneic HCT between 2003 and 2010 for AML, ALL, MDS, or CML from any donor and using any conditioning regimen were included. Starting in 2003, the CIBMTR captured data for CMV reactivation in the D100 follow-up form. A total of 15 326 patients were initially identified. The following exclusion criteria were applied based on collected patient information: information collected from National Marrow Donor Program forms that lacked posttransplant infection information (n = 3553 patients); centers with a completeness index <30% (n = 1236); information missing donor and recipient CMV serostatus (n = 488); information obtained without a signed informed consent (n = 243); and patients missing a completed D100 follow-up form (n = 203). Additional exclusion criteria based on patient demographics included use of identical twin donor (n = 67), recipients receiving multiple donor grafts (n = 45), and patient death before HCT (n = 22). Based on these collective exclusion criteria, the final study population encompassed 9469 patients. The database was locked on August 31, 2013. As a quality control, the completion of forms entered in the database for each patient was checked by the completeness index, which was 99% and 96% at 1 and 5 years of follow-up, respectively.
Data of CMV reactivation, surveillance, and treatment
Date and site of CMV reactivation and/or infection are reported by transplant centers on the D100 follow-up form. Method of testing for CMV reactivation, level of viral load as measured by quantitative PCR, and type of treatment received for CMV reactivation and/or infection are not reported to the CIBMTR.
Study design
Four disease groups of patients transplanted with bone marrow (BM)/peripheral blood (PB) were analyzed: AML (n = 5310), ALL (n = 1883), CML (n = 1079), and MDS (n = 1197). Separate analyses were performed for each disease group. First analysis was based on CMV donor and recipient (D/R) serostatus classified as D+/R+, D+/R−, D−/R+, or D−/R−. A second analysis looked at the presence or absence of CMV reactivation after transplant as a time-dependent covariate.
Variables
Variables included for analysis were as follows: CMV D/R serology (second analysis); time to CMV reactivation; HCT recipient age, sex, race, and Karnofsky score; time from hematologic diagnosis to HCT; disease risk category based on American Society for Blood and Marrow Transplantation Request for Information 2014 classification; year of transplant (2003-2006 vs 2007-2010); graft type (BM vs PB); donor type; HLA matching and donor relationship to recipient (MSD = HLA identical sibling vs MUD = well-matched unrelated donor vs other); and conditioning intensity according to CIBMTR definition, including use of total body irradiation, serotherapy with antithymoglobulin (ATG) or alemtuzumab, and T-cell depletion (TCD). Graft-versus-host disease (GVHD) prophylaxis regimen and acute (aGVHD) or chronic GVHD (cGVHD) severity were also recorded.
For multivariable analyses, the main effect variable was either D/R CMV serology (D−/R− as reference vs D+/R+ vs D−/R+ vs D+/R−) or CMV reactivation as a time dependent covariate (yes vs no). Additional variables analyzed in the models included the following: age (≤10 vs 10-30 vs >30 years); disease risk category; graft type, HLA match; conditioning intensity; serotherapy with ATG/alemtuzumab (yes vs no); GVHD prophylaxis (TCD vs tacrolimus/cyclosporine + methotrexate ± others vs tacrolimus/cyclosporine + others vs others), and aGVHD or cGVHD as a time-dependent covariate.
Statistical analysis
Patient-, disease-, and transplant-related factors were compared among groups using the Pearson χ2 test for discrete variables and the Kruskal-Wallis test for continuous variables. Probabilities of disease-free survival (DFS) and OS were calculated using the Kaplan Meier estimator. Values for other endpoints were generated using cumulative incidence estimates to account for competing risks. OS was defined as the time to death from any cause with surviving patients censored at time of last follow-up. DFS was defined as the time to relapse or death from any cause. NRM was defined as death without evidence of disease with relapse as a competing risk. Relapse was recurrence/progression of the hematologic malignancy with death as the competing risk. For aGVHD grades II to IV and cGVHD of any severity, death was the competing risk, and patients were censored at time of relapse. In both multivariable analyses of CMV serology and CMV reactivation, the proportional hazard assumption was examined. If violated, it was included as a time-dependent covariate. A stepwise selection procedure was used. Interactions between the main effect and significant covariates were examined.
Results
Patient demographics
Patient demographic information inclusive of all transplant diagnosis groups is contained in Table 1. Briefly, most patients were men between the ages of 51 and 60 years with Karnofsky performance scores >90 who received an allogeneic HCT for low-risk AML within 5 months from their leukemia diagnosis. Most patients received myeloablative (MA) conditioning without serotherapy, HLA-matched related peripheral blood mononuclear cells (PBSCs) as an allogeneic graft, and calcineurin inhibitor-based GVHD prophylaxis. Demographic information specific to each transplant diagnosis is contained in supplemental Tables 1 (AML), 2 (ALL), 3 (CML), and 4 (MDS), available on the Blood Web site.
Patient demographics
| Variable . | D/R serology, N (%) . | P value . | |||
|---|---|---|---|---|---|
| +/+ . | +/− . | −/+ . | −/− . | ||
| Patient related | |||||
| Number of patients | 3253 | 1074 | 2607 | 2535 | |
| Number of centers | 199 | 178 | 199 | 193 | |
| Age, median(range), years | 43 (1-76) | 42 (1-72) | 47 (<1-83) | 43 (1-76) | <.001 |
| Age at transplant, years | <.001 | ||||
| ≤10 | 157 (5) | 97 (9) | 145 (6) | 209 (8) | |
| 11-20 | 362 (11) | 139 (13) | 212 (8) | 262 (10) | |
| 21-30 | 408 (13) | 125 (12) | 288 (11) | 336 (13) | |
| 31-40 | 493 (15) | 135 (13) | 317 (12) | 339 (13) | |
| 41-50 | 618 (19) | 187 (17) | 529 (20) | 515 (20) | |
| 51-60 | 779 (24) | 262 (24) | 674 (26) | 570 (22) | |
| >60 | 436 (13) | 129 (12) | 442 (17) | 304 (12) | |
| Sex | <.001 | ||||
| Male | 1757 (54) | 651 (61) | 1376 (53) | 1538 (61) | |
| Female | 1496 (46) | 423 (39) | 1231 (47) | 997 (39) | |
| Karnofsky score at transplant | <.001 | ||||
| <90 | 916 (28) | 264 (25) | 813 (31) | 672 (27) | |
| ≥90 | 2230 (69) | 779 (73) | 1699 (65) | 1751 (69) | |
| Missing | 107 (3) | 31 (3) | 95 (4) | 112 (4) | |
| Race | <.001 | ||||
| Caucasian | 1866 (57) | 827 (77) | 2126 (82) | 2241 (88) | |
| African American | 192 (6) | 50 (5) | 91 (3) | 52 (2) | |
| Asian/Pacific Islander | 381 (12) | 42 (4) | 65 (2) | 27 (1) | |
| Hispanic | 450 (14) | 106 (10) | 236 (9) | 154 (6) | |
| Other | 321 (10) | 40 (4) | 55 (2) | 39 (2) | |
| Missing | 43 (1) | 9 (<1) | 34 (1) | 22 (<1) | |
| Time from diagnosis to TX, median(range), months | 7 (<1-607) | 7 (<1-275) | 7 (<1-310) | 7 (<1-314) | .189 |
| Time from diagnosis to transplant | .006 | ||||
| 0-5 mo | 1361 (42) | 474 (44) | 1140 (44) | 1163 (46) | |
| 6-11 mo | 839 (26) | 264 (25) | 599 (23) | 543 (21) | |
| 12-17 mo | 316 (10) | 106 (10) | 291 (11) | 259 (10) | |
| 18-24 mo | 208 (6) | 55 (5) | 179 (7) | 166 (7) | |
| ≥24 mo | 525 (16) | 171 (16) | 387 (15) | 393 (16) | |
| Missing | 4 (<1) | 4 (<1) | 11 (<1) | 11 (<1) | |
| Disease related | |||||
| Disease | <.001 | ||||
| AML | 1816 (56) | 583 (54) | 1594 (61) | 1317 (52) | |
| ALL | 654 (20) | 246 (23) | 470 (18) | 513 (20) | |
| CML | 436 (13) | 114 (11) | 211 (8) | 318 (13) | |
| MDS | 347 (11) | 131 (12) | 332 (13) | 387 (15) | |
| Disease risk at transplant | .006 | ||||
| Low | 1755 (54) | 599 (56) | 1317 (51) | 1388 (55) | |
| Intermediate | 706 (22) | 253 (24) | 603 (23) | 531 (21) | |
| High | 785 (24) | 222 (21) | 684 (26) | 612 (24) | |
| Missing | 7 (<1) | 0 | 3 (<1) | 4 (<1) | |
| Transplant related | |||||
| CMV reactivation in blood by 1 year | <.001 | ||||
| No | 2211 (68) | 958 (89) | 1763 (68) | 2432 (96) | |
| Yes | 1032 (32) | 115 (11) | 841 (32) | 99 (4) | |
| Missing | 10 (<1) | 1 (<1) | 3 (<1) | 4 (<1) | |
| Time from TX to CMV reactivation in blood, median (range), days | 41 (1-359) | 46 (7-235) | 38 (1-362) | 45 (6-350) | .003 |
| Graft type | <.001 | ||||
| Bone marrow | 858 (26) | 302 (28) | 604 (23) | 711 (28) | |
| Peripheral blood | 2395 (74) | 772 (72) | 2003 (77) | 1824 (72) | |
| Donor/recipient HLA match | <.001 | ||||
| HLA-identical siblings | 1816 (56) | 428 (40) | 856 (33) | 971 (38) | |
| Other related | 199 (6) | 61 (6) | 106 (4) | 115 (5) | |
| Well-matched unrelated | 800 (25) | 386 (36) | 1190 (46) | 1105 (44) | |
| Partially matched unrelated | 316 (10) | 158 (15) | 357 (14) | 283 (11) | |
| Mismatched unrelated | 115 (4) | 38 (4) | 89 (3) | 51 (2) | |
| Unrelated (HLA match information missing) | 7 (<1) | 3 (<1) | 9 (<1) | 9 (<1) | |
| Missing | 0 | 0 | 0 | 1 (<1) | |
| Conditioning regimen intensity | <.001 | ||||
| MA | 2421 (74) | 809 (75) | 1940 (74) | 2001 (79) | |
| Non-MA/RIC | 827 (25) | 265 (25) | 667 (26) | 532 (21) | |
| Missing | 5 (<1) | 0 | 0 | 2 (<1) | |
| ATG/CAMPATH as conditioning or GVHD prophylaxis | <.001 | ||||
| ATG + CAMPATH | 0 | 0 | 1 (<1) | 0 | |
| ATG alone | 735 (23) | 262 (24) | 703 (27) | 631 (25) | |
| CAMPATH alone | 116 (4) | 58 (5) | 113 (4) | 82 (3) | |
| No ATG or CAMPATH | 2395 (74) | 752 (70) | 1789 (69) | 1822 (72) | |
| Missing | 7 (<1) | 2 (<1) | 1 (<1) | 0 | |
| GVHD prophylaxis | <.001 | ||||
| Both in vivo and ex vivo | 62 (2) | 27 (3) | 46 (2) | 63 (2) | |
| In vivo only | 789 (24) | 293 (27) | 771 (30) | 650 (26) | |
| Ex vivo only | 112 (3) | 28 (3) | 61 (2) | 82 (3) | |
| Post TX cyclophosphamide + others | 19 (<1) | 12 (1) | 18 (<1) | 27 (1) | |
| FK506 + MMF +- others | 190 (6) | 82 (8) | 220 (8) | 194 (8) | |
| FK506 + MTX +- others (except MMF) | 840 (26) | 309 (29) | 848 (33) | 792 (31) | |
| FK506 + others (except MTX, MMF) | 152 (5) | 48 (4) | 116 (4) | 99 (4) | |
| CSA + MMF +- others (except FK506) | 120 (4) | 36 (3) | 81 (3) | 81 (3) | |
| CSA + MTX +- others (except FK506, MMF) | 856 (26) | 200 (19) | 391 (15) | 462 (18) | |
| CSA + others (except FK506, MTX, MMF) | 72 (2) | 24 (2) | 38 (1) | 55 (2) | |
| Other GVHD prophylaxis | 41 (1) | 15 (1) | 17 (<1) | 30 (1) | |
| aGVHD | |||||
| 0-I | 2075 (64) | 652 (61) | 1580 (61) | 1525 (60) | |
| II-IV | 1143 (35) | 411 (38) | 1004 (39) | 981 (39) | |
| Missing | 35 (1) | 11 (1) | 23 (<1) | 29 (1) | |
| Time from TX to aGVHD, median (range), months | 1 (<1-22) | 1 (<1-6) | 1 (<1-6) | 1 (<1-29) | |
| cGVHD | |||||
| No | 1755 (54) | 527 (49) | 1345 (52) | 1250 (49) | |
| Yes | 1418 (44) | 523 (49) | 1206 (46) | 1230 (48) | |
| Missing | 80 (2) | 24 (2) | 56 (2) | 55 (2) | |
| Time from TX to cGVHD, median (range), months | 5 (2-68) | 6 (2-51) | 5 (2-72) | 6 (2-49) | |
| Year of transplant | <.001 | ||||
| 2003 | 336 (10) | 73 (7) | 148 (6) | 143 (6) | |
| 2004 | 387 (12) | 83 (8) | 183 (7) | 202 (8) | |
| 2005 | 444 (14) | 111 (10) | 236 (9) | 255 (10) | |
| 2006 | 457 (14) | 165 (15) | 355 (14) | 346 (14) | |
| 2007 | 332 (10) | 138 (13) | 379 (15) | 344 (14) | |
| 2008 | 547 (17) | 199 (19) | 467 (18) | 518 (20) | |
| 2009 | 438 (13) | 181 (17) | 498 (19) | 439 (17) | |
| 2010 | 312 (10) | 124 (12) | 341 (13) | 288 (11) | |
| Median follow-up of survivors, months | 61 (1-125) | 59 (3-121) | 60 (2-127) | 60 (3-124) | |
| Variable . | D/R serology, N (%) . | P value . | |||
|---|---|---|---|---|---|
| +/+ . | +/− . | −/+ . | −/− . | ||
| Patient related | |||||
| Number of patients | 3253 | 1074 | 2607 | 2535 | |
| Number of centers | 199 | 178 | 199 | 193 | |
| Age, median(range), years | 43 (1-76) | 42 (1-72) | 47 (<1-83) | 43 (1-76) | <.001 |
| Age at transplant, years | <.001 | ||||
| ≤10 | 157 (5) | 97 (9) | 145 (6) | 209 (8) | |
| 11-20 | 362 (11) | 139 (13) | 212 (8) | 262 (10) | |
| 21-30 | 408 (13) | 125 (12) | 288 (11) | 336 (13) | |
| 31-40 | 493 (15) | 135 (13) | 317 (12) | 339 (13) | |
| 41-50 | 618 (19) | 187 (17) | 529 (20) | 515 (20) | |
| 51-60 | 779 (24) | 262 (24) | 674 (26) | 570 (22) | |
| >60 | 436 (13) | 129 (12) | 442 (17) | 304 (12) | |
| Sex | <.001 | ||||
| Male | 1757 (54) | 651 (61) | 1376 (53) | 1538 (61) | |
| Female | 1496 (46) | 423 (39) | 1231 (47) | 997 (39) | |
| Karnofsky score at transplant | <.001 | ||||
| <90 | 916 (28) | 264 (25) | 813 (31) | 672 (27) | |
| ≥90 | 2230 (69) | 779 (73) | 1699 (65) | 1751 (69) | |
| Missing | 107 (3) | 31 (3) | 95 (4) | 112 (4) | |
| Race | <.001 | ||||
| Caucasian | 1866 (57) | 827 (77) | 2126 (82) | 2241 (88) | |
| African American | 192 (6) | 50 (5) | 91 (3) | 52 (2) | |
| Asian/Pacific Islander | 381 (12) | 42 (4) | 65 (2) | 27 (1) | |
| Hispanic | 450 (14) | 106 (10) | 236 (9) | 154 (6) | |
| Other | 321 (10) | 40 (4) | 55 (2) | 39 (2) | |
| Missing | 43 (1) | 9 (<1) | 34 (1) | 22 (<1) | |
| Time from diagnosis to TX, median(range), months | 7 (<1-607) | 7 (<1-275) | 7 (<1-310) | 7 (<1-314) | .189 |
| Time from diagnosis to transplant | .006 | ||||
| 0-5 mo | 1361 (42) | 474 (44) | 1140 (44) | 1163 (46) | |
| 6-11 mo | 839 (26) | 264 (25) | 599 (23) | 543 (21) | |
| 12-17 mo | 316 (10) | 106 (10) | 291 (11) | 259 (10) | |
| 18-24 mo | 208 (6) | 55 (5) | 179 (7) | 166 (7) | |
| ≥24 mo | 525 (16) | 171 (16) | 387 (15) | 393 (16) | |
| Missing | 4 (<1) | 4 (<1) | 11 (<1) | 11 (<1) | |
| Disease related | |||||
| Disease | <.001 | ||||
| AML | 1816 (56) | 583 (54) | 1594 (61) | 1317 (52) | |
| ALL | 654 (20) | 246 (23) | 470 (18) | 513 (20) | |
| CML | 436 (13) | 114 (11) | 211 (8) | 318 (13) | |
| MDS | 347 (11) | 131 (12) | 332 (13) | 387 (15) | |
| Disease risk at transplant | .006 | ||||
| Low | 1755 (54) | 599 (56) | 1317 (51) | 1388 (55) | |
| Intermediate | 706 (22) | 253 (24) | 603 (23) | 531 (21) | |
| High | 785 (24) | 222 (21) | 684 (26) | 612 (24) | |
| Missing | 7 (<1) | 0 | 3 (<1) | 4 (<1) | |
| Transplant related | |||||
| CMV reactivation in blood by 1 year | <.001 | ||||
| No | 2211 (68) | 958 (89) | 1763 (68) | 2432 (96) | |
| Yes | 1032 (32) | 115 (11) | 841 (32) | 99 (4) | |
| Missing | 10 (<1) | 1 (<1) | 3 (<1) | 4 (<1) | |
| Time from TX to CMV reactivation in blood, median (range), days | 41 (1-359) | 46 (7-235) | 38 (1-362) | 45 (6-350) | .003 |
| Graft type | <.001 | ||||
| Bone marrow | 858 (26) | 302 (28) | 604 (23) | 711 (28) | |
| Peripheral blood | 2395 (74) | 772 (72) | 2003 (77) | 1824 (72) | |
| Donor/recipient HLA match | <.001 | ||||
| HLA-identical siblings | 1816 (56) | 428 (40) | 856 (33) | 971 (38) | |
| Other related | 199 (6) | 61 (6) | 106 (4) | 115 (5) | |
| Well-matched unrelated | 800 (25) | 386 (36) | 1190 (46) | 1105 (44) | |
| Partially matched unrelated | 316 (10) | 158 (15) | 357 (14) | 283 (11) | |
| Mismatched unrelated | 115 (4) | 38 (4) | 89 (3) | 51 (2) | |
| Unrelated (HLA match information missing) | 7 (<1) | 3 (<1) | 9 (<1) | 9 (<1) | |
| Missing | 0 | 0 | 0 | 1 (<1) | |
| Conditioning regimen intensity | <.001 | ||||
| MA | 2421 (74) | 809 (75) | 1940 (74) | 2001 (79) | |
| Non-MA/RIC | 827 (25) | 265 (25) | 667 (26) | 532 (21) | |
| Missing | 5 (<1) | 0 | 0 | 2 (<1) | |
| ATG/CAMPATH as conditioning or GVHD prophylaxis | <.001 | ||||
| ATG + CAMPATH | 0 | 0 | 1 (<1) | 0 | |
| ATG alone | 735 (23) | 262 (24) | 703 (27) | 631 (25) | |
| CAMPATH alone | 116 (4) | 58 (5) | 113 (4) | 82 (3) | |
| No ATG or CAMPATH | 2395 (74) | 752 (70) | 1789 (69) | 1822 (72) | |
| Missing | 7 (<1) | 2 (<1) | 1 (<1) | 0 | |
| GVHD prophylaxis | <.001 | ||||
| Both in vivo and ex vivo | 62 (2) | 27 (3) | 46 (2) | 63 (2) | |
| In vivo only | 789 (24) | 293 (27) | 771 (30) | 650 (26) | |
| Ex vivo only | 112 (3) | 28 (3) | 61 (2) | 82 (3) | |
| Post TX cyclophosphamide + others | 19 (<1) | 12 (1) | 18 (<1) | 27 (1) | |
| FK506 + MMF +- others | 190 (6) | 82 (8) | 220 (8) | 194 (8) | |
| FK506 + MTX +- others (except MMF) | 840 (26) | 309 (29) | 848 (33) | 792 (31) | |
| FK506 + others (except MTX, MMF) | 152 (5) | 48 (4) | 116 (4) | 99 (4) | |
| CSA + MMF +- others (except FK506) | 120 (4) | 36 (3) | 81 (3) | 81 (3) | |
| CSA + MTX +- others (except FK506, MMF) | 856 (26) | 200 (19) | 391 (15) | 462 (18) | |
| CSA + others (except FK506, MTX, MMF) | 72 (2) | 24 (2) | 38 (1) | 55 (2) | |
| Other GVHD prophylaxis | 41 (1) | 15 (1) | 17 (<1) | 30 (1) | |
| aGVHD | |||||
| 0-I | 2075 (64) | 652 (61) | 1580 (61) | 1525 (60) | |
| II-IV | 1143 (35) | 411 (38) | 1004 (39) | 981 (39) | |
| Missing | 35 (1) | 11 (1) | 23 (<1) | 29 (1) | |
| Time from TX to aGVHD, median (range), months | 1 (<1-22) | 1 (<1-6) | 1 (<1-6) | 1 (<1-29) | |
| cGVHD | |||||
| No | 1755 (54) | 527 (49) | 1345 (52) | 1250 (49) | |
| Yes | 1418 (44) | 523 (49) | 1206 (46) | 1230 (48) | |
| Missing | 80 (2) | 24 (2) | 56 (2) | 55 (2) | |
| Time from TX to cGVHD, median (range), months | 5 (2-68) | 6 (2-51) | 5 (2-72) | 6 (2-49) | |
| Year of transplant | <.001 | ||||
| 2003 | 336 (10) | 73 (7) | 148 (6) | 143 (6) | |
| 2004 | 387 (12) | 83 (8) | 183 (7) | 202 (8) | |
| 2005 | 444 (14) | 111 (10) | 236 (9) | 255 (10) | |
| 2006 | 457 (14) | 165 (15) | 355 (14) | 346 (14) | |
| 2007 | 332 (10) | 138 (13) | 379 (15) | 344 (14) | |
| 2008 | 547 (17) | 199 (19) | 467 (18) | 518 (20) | |
| 2009 | 438 (13) | 181 (17) | 498 (19) | 439 (17) | |
| 2010 | 312 (10) | 124 (12) | 341 (13) | 288 (11) | |
| Median follow-up of survivors, months | 61 (1-125) | 59 (3-121) | 60 (2-127) | 60 (3-124) | |
The Pearson χ2 test was used for comparing discrete variables; the Kruskal-Wallis test was used for comparing continuous variables. FK506, tacrolimus; MMF, mycophenolate mofetil; TBI, total body irradiation; TX, transplant.
Distribution of CMV serology and CMV reactivation
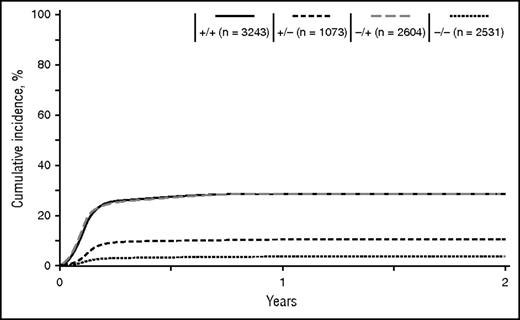
Distribution of donor and recipient CMV serology was consistent throughout the different disease categories (Tables 1 and 2). For the entire cohort, the D+/R+ group was the most prevalent at 34% (range, 29-40%), followed by D−/R+ at 28% (20-30%), D−/R− at 27% (25−32%), and D+/R− at 11% (11-13%). The median time to CMV reactivation was 41 days (range, 1-362 days) after HCT, and nearly all reactivations (98%) occurred before D100 (Figure 1). As expected, the incidence of reactivation was higher for D−/R+ (34%) and D+/R+ (32%) groups, whereas the D+/R− group was protected against reactivation (11%). Reactivation among D−/R− (4%) was attributed to either false-negative serology or primary infection in the peritransplant period.
Distribution of CMV serology and CMV reactivation at 1 year after HCT
| . | D/R serology . | Overall P value . | |||
|---|---|---|---|---|---|
| +/+ . | +/− . | −/+ . | −/− . | ||
| AML BM/PBSC, N (%) | 1816 (34) | 583 (11) | 1594 (30) | 1317 (25) | |
| CMV reactivation by 1 year | <.001 | ||||
| No | 1249 (69) | 511 (88) | 1085 (68) | 1256 (95) | |
| Yes | 565 (31) | 72 (12) | 507 (32) | 59 (4) | |
| Missing | 2 (<1) | 0 | 2 (<1) | 2 (<1) | |
| Days from TX to reactivation | 40 (1-355) | 46 (7-235) | 36 (2-337) | 44 (11-350) | .01 |
| ALL BM/PBSC, N (%) | 654 (35) | 246 (13) | 470 (25) | 513 (27) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 440 (67) | 231 (94) | 352 (75) | 490 (96) | |
| Yes | 210 (32) | 15 (6) | 118 (25) | 22 (4) | |
| Missing | 4 (<1) | 0 | 0 | 1 (<1) | |
| Days from TX to reactivation | 39 (4-359) | 47 (23-92) | 34 (2-329) | 41 (6-231) | .242 |
| CML BM/PBSC, N (%) | 436 (40) | 114 (11) | 211 (20) | 318 (29) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 296 (68) | 97 (85) | 126 (60) | 310 (97) | |
| Yes | 138 (32) | 16 (14) | 84 (40) | 7 (2) | |
| Missing | 2 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | |
| No | 48 (1-264) | 57 (8-83) | 49 (4-330) | 53 (10-71) | .637 |
| MDS BM/PBSC, N (%) | 347 (29) | 131 (11) | 332 (28) | 387 (32) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 226 (65) | 119 (91) | 200 (60) | 376 (97) | |
| Yes | 119 (34) | 12 (9) | 132 (40) | 11 (3) | |
| Missing | 2 (<1) | 0 | 0 | 0 | |
| Days from TX to reactivation | 46 (1-322) | 43 (22-128) | 42 (1-362) | 70 (28-336) | .117 |
| . | D/R serology . | Overall P value . | |||
|---|---|---|---|---|---|
| +/+ . | +/− . | −/+ . | −/− . | ||
| AML BM/PBSC, N (%) | 1816 (34) | 583 (11) | 1594 (30) | 1317 (25) | |
| CMV reactivation by 1 year | <.001 | ||||
| No | 1249 (69) | 511 (88) | 1085 (68) | 1256 (95) | |
| Yes | 565 (31) | 72 (12) | 507 (32) | 59 (4) | |
| Missing | 2 (<1) | 0 | 2 (<1) | 2 (<1) | |
| Days from TX to reactivation | 40 (1-355) | 46 (7-235) | 36 (2-337) | 44 (11-350) | .01 |
| ALL BM/PBSC, N (%) | 654 (35) | 246 (13) | 470 (25) | 513 (27) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 440 (67) | 231 (94) | 352 (75) | 490 (96) | |
| Yes | 210 (32) | 15 (6) | 118 (25) | 22 (4) | |
| Missing | 4 (<1) | 0 | 0 | 1 (<1) | |
| Days from TX to reactivation | 39 (4-359) | 47 (23-92) | 34 (2-329) | 41 (6-231) | .242 |
| CML BM/PBSC, N (%) | 436 (40) | 114 (11) | 211 (20) | 318 (29) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 296 (68) | 97 (85) | 126 (60) | 310 (97) | |
| Yes | 138 (32) | 16 (14) | 84 (40) | 7 (2) | |
| Missing | 2 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | |
| No | 48 (1-264) | 57 (8-83) | 49 (4-330) | 53 (10-71) | .637 |
| MDS BM/PBSC, N (%) | 347 (29) | 131 (11) | 332 (28) | 387 (32) | |
| CMV reactivation by 1 y | <.001 | ||||
| No | 226 (65) | 119 (91) | 200 (60) | 376 (97) | |
| Yes | 119 (34) | 12 (9) | 132 (40) | 11 (3) | |
| Missing | 2 (<1) | 0 | 0 | 0 | |
| Days from TX to reactivation | 46 (1-322) | 43 (22-128) | 42 (1-362) | 70 (28-336) | .117 |
Time from TX to reactivation expressed as mean (range). N, number of patients.
Cumulative incidence curves for CMV reactivation according to D/R serology.
Hematologic disease relapse
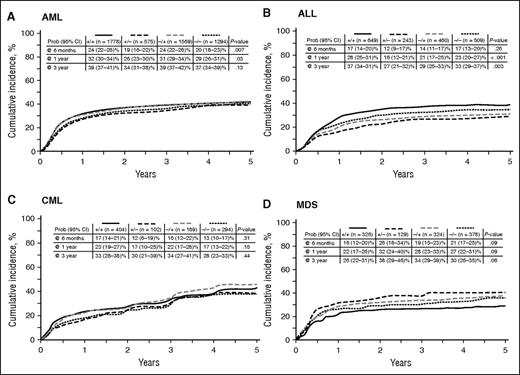
In univariate analysis, the risk of AML relapse at 6 months was 24% (95% confidence interval [CI], 22-26%) for D+/R+ and D−/R+, which was significantly higher than in the D−/R− cohort (20%; 95% CI, 18-23%; P = .007; Figure 2A). However, no difference in AML relapse according to CMV serology was noted at 3 years after HCT (Table 3). For patients with ALL, the incidence of relapse was significantly lower in the D+/R− cohort at 1 year (16%; 95% CI, 12-21%; P <.001) and was maintained at 3 years of follow-up (27%; 95% CI, 21-32%; P = .003; Figure 2B; Table 3).
Cumulative incidence curves for indicated hematologic disease relapse according to D/R CMV serology.
Cumulative incidence curves for indicated hematologic disease relapse according to D/R CMV serology.
Univariate analysis of transplant outcomes by disease and donor/recipient CMV serology
| Disease . | D+/R+ [% (95% CI)] . | D+/R− [% (95% CI)] . | D−/R+ [% (95% CI)] . | D−/R− [% (95% CI)] . | P value . |
|---|---|---|---|---|---|
| Relapse at 3 years | |||||
| AML | 39 (37- 41)% | 34 (31-38)% | 39 (37-42)% | 37 (34-39)% | .13 |
| ALL | 37 (34-41)% | 27 (21-32)% | 29 (25-33)% | 33 (29-37)% | .003 |
| CML | 33 (28-38)% | 30 (21-39)% | 34 (27-41)% | 28 (23-33)% | .44 |
| MDS | 26 (22-31)% | 38 (29-46)% | 34 (29-39)% | 30 (26-35)% | .06 |
| NRM at 3 years | |||||
| AML | 18 (16-20)% | 21 (18-24)% | 20 (18-22)% | 15 (13-17)% | <.001 |
| ALL | 25 (22-29)% | 18 (14-23)% | 22 (18-26)% | 16 (13-19)% | <.001 |
| CML | 17 (14-21)% | 15 (9-22)% | 15 (11-21)% | 14 (10-18)% | .64 |
| MDS | 28 (23-33)% | 23 (16-31)% | 23 (19-28)% | 23 (19-27)% | .49 |
| OS at 3 years | |||||
| AML | 47 (45-49)% | 51 (46-55)% | 45 (43-48)% | 54 (51-56)% | <.001 |
| ALL | 44 (40-48)% | 59 (53-65)% | 54 (50-59)% | 60 (56-64)% | <.001 |
| CML | 61 (56-66)% | 66 (57-74)% | 61 (55-68)% | 70 (64-75)% | .08 |
| MDS | 48 (43-54)% | 46 (37-55)% | 47 (41-52)% | 51 (45-56)% | .74 |
| Disease-free survival at 3 years | |||||
| AML | 43 (41-45)% | 45 (41-49)% | 41 (38-43)% | 49 (46-52)% | <.001 |
| ALL | 37 (34-41)% | 55 (49-61)% | 49 (45-54)% | 52 (47-56)% | <.001 |
| CML | 50 (45-55)% | 56 (46-65)% | 51 (44-58)% | 58 (53-64)% | .13 |
| MDS | 46 (41-51%) | 39 (30-48)% | 43 (37-48) | 47 (42-52)% | .036 |
| aGVHD at 1 year | |||||
| AML | 34(32-37)% | 35 (31-39)% | 37 (35-39)% | 38 (36-41)% | .11 |
| ALL | 37 (33-41)% | 42 (35-48)% | 39 (35-44)% | 34 (30-38)% | .16 |
| CML | 35 (30-39)% | 43 (34-52)% | 48 (41-55)% | 45 (39-50)% | .004 |
| MDS | 41 (36-46)% | 46 (37-55)% | 42 (37-48)% | 45(40-50)% | .66 |
| cGVHD at 3 years | |||||
| AML | 44 (42-46)% | 50 (46-54)% | 47 (44-49)% | 51 (48-54)% | <.001 |
| ALL | 46 (42-50)% | 51 (45-57)% | 46 (41-50)% | 43 (38-47)% | .18 |
| CML | 51 (46-56)% | 49 (39-58)% | 56 (48-62)% | 44 (49-60)% | .57 |
| MDS | 48 (43-54)% | 49 (40-58)% | 50 (45-56)% | 52 (47-57)% | .76 |
| Disease . | D+/R+ [% (95% CI)] . | D+/R− [% (95% CI)] . | D−/R+ [% (95% CI)] . | D−/R− [% (95% CI)] . | P value . |
|---|---|---|---|---|---|
| Relapse at 3 years | |||||
| AML | 39 (37- 41)% | 34 (31-38)% | 39 (37-42)% | 37 (34-39)% | .13 |
| ALL | 37 (34-41)% | 27 (21-32)% | 29 (25-33)% | 33 (29-37)% | .003 |
| CML | 33 (28-38)% | 30 (21-39)% | 34 (27-41)% | 28 (23-33)% | .44 |
| MDS | 26 (22-31)% | 38 (29-46)% | 34 (29-39)% | 30 (26-35)% | .06 |
| NRM at 3 years | |||||
| AML | 18 (16-20)% | 21 (18-24)% | 20 (18-22)% | 15 (13-17)% | <.001 |
| ALL | 25 (22-29)% | 18 (14-23)% | 22 (18-26)% | 16 (13-19)% | <.001 |
| CML | 17 (14-21)% | 15 (9-22)% | 15 (11-21)% | 14 (10-18)% | .64 |
| MDS | 28 (23-33)% | 23 (16-31)% | 23 (19-28)% | 23 (19-27)% | .49 |
| OS at 3 years | |||||
| AML | 47 (45-49)% | 51 (46-55)% | 45 (43-48)% | 54 (51-56)% | <.001 |
| ALL | 44 (40-48)% | 59 (53-65)% | 54 (50-59)% | 60 (56-64)% | <.001 |
| CML | 61 (56-66)% | 66 (57-74)% | 61 (55-68)% | 70 (64-75)% | .08 |
| MDS | 48 (43-54)% | 46 (37-55)% | 47 (41-52)% | 51 (45-56)% | .74 |
| Disease-free survival at 3 years | |||||
| AML | 43 (41-45)% | 45 (41-49)% | 41 (38-43)% | 49 (46-52)% | <.001 |
| ALL | 37 (34-41)% | 55 (49-61)% | 49 (45-54)% | 52 (47-56)% | <.001 |
| CML | 50 (45-55)% | 56 (46-65)% | 51 (44-58)% | 58 (53-64)% | .13 |
| MDS | 46 (41-51%) | 39 (30-48)% | 43 (37-48) | 47 (42-52)% | .036 |
| aGVHD at 1 year | |||||
| AML | 34(32-37)% | 35 (31-39)% | 37 (35-39)% | 38 (36-41)% | .11 |
| ALL | 37 (33-41)% | 42 (35-48)% | 39 (35-44)% | 34 (30-38)% | .16 |
| CML | 35 (30-39)% | 43 (34-52)% | 48 (41-55)% | 45 (39-50)% | .004 |
| MDS | 41 (36-46)% | 46 (37-55)% | 42 (37-48)% | 45(40-50)% | .66 |
| cGVHD at 3 years | |||||
| AML | 44 (42-46)% | 50 (46-54)% | 47 (44-49)% | 51 (48-54)% | <.001 |
| ALL | 46 (42-50)% | 51 (45-57)% | 46 (41-50)% | 43 (38-47)% | .18 |
| CML | 51 (46-56)% | 49 (39-58)% | 56 (48-62)% | 44 (49-60)% | .57 |
| MDS | 48 (43-54)% | 49 (40-58)% | 50 (45-56)% | 52 (47-57)% | .76 |
P values with statistical significance (P < .05) are noted in bold type.
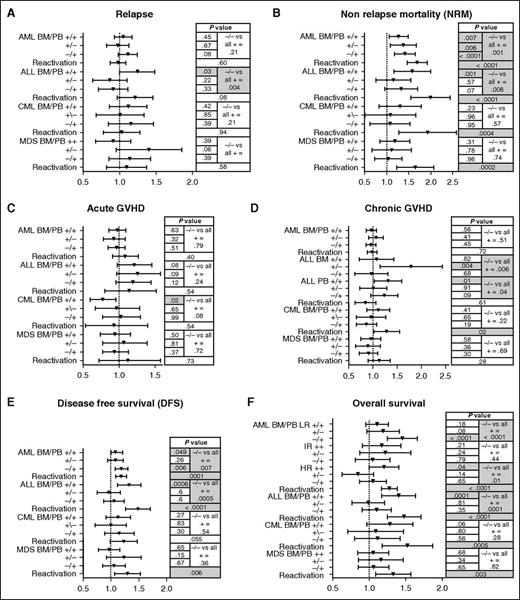
In multivariate analysis, there was no impact of D/R CMV serology on relapse risk for patients with AML, CML, or MDS (Figure 3A). Paradoxically, for ALL patients, positive D/R serology increased relapse risk (any D/R vs D−/R−, P = .004). This higher relapse risk was limited to the D+/R+ group, which had higher relative risk (RR) for relapse compared with D−/R− (RR, 1.22; 95% CI, 1.01-1.49; P = .038), with D−/R+ (RR, 1.37; 95% CI, 1.11-1.68; P = .003), and with D+/R− (RR, 1.45; 95% CI, 1.11-1.89; P = .005). In any disease group, CMV reactivation as a time-dependent covariate was not associated with disease relapse (Figure 3A).
Multivariable analysis of risk factors for outcomes depending on CMV donor/recipient serology or CMV reactivation. (A) Relapse, (B) NRM, (C) aGVHD, (D) cGVHD, (E) DFS, and (F) OS. For multivariate analysis, D−/R− = 1, and no CMV reactivation = 1.
Multivariable analysis of risk factors for outcomes depending on CMV donor/recipient serology or CMV reactivation. (A) Relapse, (B) NRM, (C) aGVHD, (D) cGVHD, (E) DFS, and (F) OS. For multivariate analysis, D−/R− = 1, and no CMV reactivation = 1.
Multivariate analysis also found several classical risk factors for disease relapse. For example, higher-risk hematologic disease increased relapse risk in all disease categories (AML high RR, 5.3; 95% CI, 3.9-7.3); AML intermediate [int] RR, 1.9; 95% CI, 1.5-2.3; P <.0001; ALL high RR, 1.9; 95% CI, 1.3-2.6, ALL int RR, 1.3; 95% CI, 1.0-1.6; P = .0012; CML high RR, 5.3; 95% CI, 3.9-7.3; CML int RR, 1.9; 95% CI, 1.5-2.3; P <.001; MDS high RR, 1.84; 95% CI, 1.5-2.3; P <.0001). Reduced intensity conditioning regimen (RIC) was associated with AML relapse (RR, 1.3; 95% CI, 1.2-1.5; P <.0001) and MDS (RR, 1.4; 95% CI, 1.16-1.75; P = .0008). Anti–T-cell therapy (ATG or alemtuzumab) was a risk factor for relapse in patients with CML (RR, 1.5; 95% CI, 1.2-1.8; P = .0059) and MDS (RR, 1.3; 95% CI, 1.1-1.6; P = .008). For patients with ALL, older age (>30 years) (RR, 1.5; 95% CI, 1.14-1.91 [11-30 years: RR, 1.3; 95% CI, 0.98-1.6; P = .009) was associated with relapse.
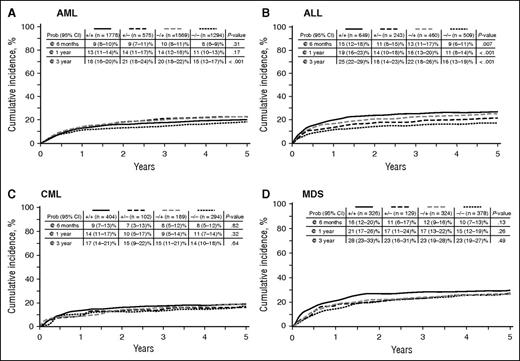
NRM
Contrasting with its limited effect in hematologic disease relapse, CMV was strongly associated with NRM. In univariate analysis, positive D/R serology (any D/R vs D−/R−) was a risk factor for NRM among AML and ALL patients but not CML or MDS patients (Table 3; Figure 4A-D). In multivariate analysis, positive CMV serology increased the risk of NRM for AML and ALL patients but had no effect for CML or MDS patients (Figure 3B). Increased NRM was observed for all serology groups (D+/R+, D+/R−, D−/R+) compared with D−/R−. CMV reactivation also resulted in higher NRM for all disease groups, with risks ranging from RR = 1.61 (95% CI, 1.25-2.08) for MDS to RR = 1.95 (95% CI, 1.56-2.45) for ALL (Figure 3B).
Cumulative incidence curves for NRM by indicated hematologic disease and according to D/R CMV serology.
Cumulative incidence curves for NRM by indicated hematologic disease and according to D/R CMV serology.
Older age at transplant increased NRM regardless of disease. Other risk factors for increased NRM in patients with AML, ALL, and CML included higher disease risk category, HLA incompatibility, and use of PBSC graft. For patients with AML and CML, increased NRM occurred in patients receiving MA conditioning. Development of aGVHD increased NRM for patients with ALL but was not significant in other diseases. For patients with AML, use of TCD as GVHD prophylaxis was associated with greater NRM. However, use of anti–T-cell serotherapy was associated with less NRM beyond 10 months from transplant.
GVHD
In univariate analysis, incidence of grade II to IV aGVHD by D100 was similar across the 4 D/R serology cohorts regardless of disease, with the exception of a lower incidence for the CML D+/R+ cohort (Table 3). Multivariate analysis indicated D+/R+ serology for CML patients was also associated with decreased incidence of aGVHD, whereas neither D/R CMV serology nor CMV reactivation as a time-dependent covariate affected incidence of grade II to IV aGVHD in any other group (Figure 3C). Other risk factors for grade II to IV aGVHD by multivariate analysis were older age, donor other than MSD, more intense conditioning regimen, absence of serotherapy, less intense GVHD prophylaxis (TCD vs prophylaxis without TCD), and the use of PBSCs.
For cGVHD, regardless of severity, the univariate analysis did not show any association with CMV serology except for AML where the incidence was lower in the D+/R+ cohort (Table 3). However, multivariate analysis did not reveal any influence of CMV serology or reactivation on cGVHD in any disease group (Figure 3D). Regarding graft stem cell source, an association between CMV serology and cGVHD was found for ALL in D+/R− patients transplanted with BM whom had a 1.7-fold (95% CI, 1.188-2.480; P = .004) RR for cGVHD compared with the D−/R− cohort and 1.2-fold RR of cGVHD in the subgroup of D+/R+ transplanted with PBSCs (95% CI, 0.969-1.527; P = .091) compared with D−/R−. Finally, CMV reactivation did not affect the incidence of cGVHD. Risk factors associated with cGVHD by multivariate analysis were older recipient age at HCT (>10 years), donor use other than MSD, previous diagnosis of aGVHD, more intense conditioning regimen, absence of serotherapy, less intense GVHD prophylaxis, and use of a PBSC graft.
DFS
In univariate analysis, D/R CMV seropositive status was associated with worse 3-year DFS for patients with ALL, AML, and MDS, but not for patients with CML (Table 3). In multivariate analysis, recipient CMV positive serology was associated with inferior DFS for patients with AML and ALL (Figure 3E). Moreover, CMV reactivation was strongly associated with inferior DFS among AML, ALL, and MDS (Figure 3E). Other risk factors identified in multivariate analysis for lower DFS were older transplant recipient age, higher disease risk category, HLA incompatibility, PBSC graft, less intensive conditioning regimen, and absence of TCD serotherapy. DFS was higher among MDS with GVHD prophylaxis involving TCD. Patients with CML had better DFS associated with a less intensive conditioning regimen. AML and ALL had better DFS with MUD vs MSD (RR, 0.90; 95% CI, 0.80-0.98; P = .02 and RR, 0.8; 95% CI, 0.63-0.93; P = .004, respectively).
OS
Higher NRM resulted in lower OS among transplant recipients with AML and ALL, but not those with CML and MDS (Tables 3; Figure 3F). In multivariate analysis, positive CMV serology associating with lower OS was also found in AML, but was limited to low risk (P < .0001) and high risk (P = .013) disease. Lower OS was also observed in D+/R+ patients with high-risk disease (RR, 1.19; 95% CI, 1.00-1.41; P = .04) and in the D−/R+ group with low-risk disease (RR, 1.44; 95% CI, 1.25-1.66; P < .0001). Positive CMV serology and lower OS were also noted in HCT recipients with ALL (P = .0001; Figure 3F). In ALL, increased risk of death was correlated with D+/R+ (RR, 1.39; 95% CI, 1.17-1.64; P = .0001). Last, CMV reactivation was associated with inferior OS among all disease groups in multivariate analysis (AML: RR, 1.27; 95% CI, 1.17-1.38; P < .0001; ALL: RR, 1.46; 95% CI, 1.25-1.7; P <.0001; CML: RR, 1.49; 95% CI, 1.19-1.88; P = .0005; MDS: RR, 1.31; 95% CI, 1.09-1.57; P = .003; Figure 3F). Risk factors for inferior OS were older transplant recipient age and higher disease risk throughout all disease groups. In contrast, MUD conferred higher OS compared with MSD or other donors for AML and ALL, whereas MSD conferred higher OS for CML and MDS. aGVHD and PBSC graft were associated with decreased OS for ALL, and TCD conditioning worsened OS for CML.
Table 4 contains information on cause of death (n = 5057 patients, 53.4%). The top 4 causes of death were recurrent/refractory primary disease (n = 2211, 43.7%), infection (n = 787, 15.6%), organ failure (n = 700, 13.8%), and GVHD (n = 597, 11.8%).
Cause of death following allogeneic HCT
| . | D+/R+ . | D+/R− . | D−/R+ . | D−/R− . | P value . |
|---|---|---|---|---|---|
| Status | |||||
| Alive | 1452 (45) | 527 (49) | 1131 (43) | 1302 (51) | |
| Dead | 1801 (55) | 547 (51) | 1476 (57) | 1233 (49) | |
| Cause of death | .404 | ||||
| Graft rejection | 13 (<1) | 5 (<1) | 14 (<1) | 9 (<1) | |
| Infection | 273 (15) | 94 (17) | 237 (16) | 183 (15) | |
| IPN | 26 (1) | 7 (1) | 22 (1) | 16 (1) | |
| Organ failure | 281 (16) | 73 (13) | 195 (13) | 151 (12) | |
| GVHD | 210 (12) | 73 (13) | 165 (11) | 149 (12) | |
| Recurrent/persistent disease | 758 (42) | 224 (41) | 660 (45) | 569 (46) | |
| Secondary malignancy | 16 (<1) | 8 (1) | 23 (2) | 13 (1) | |
| Hemorrhage | 39 (2) | 8 (1) | 20 (1) | 27 (2) | |
| Other cause | 117 (6) | 35 (6) | 99 (7) | 85 (7) | |
| Unknown | 68 (4) | 20 (4) | 41 (3) | 31 (3) |
| . | D+/R+ . | D+/R− . | D−/R+ . | D−/R− . | P value . |
|---|---|---|---|---|---|
| Status | |||||
| Alive | 1452 (45) | 527 (49) | 1131 (43) | 1302 (51) | |
| Dead | 1801 (55) | 547 (51) | 1476 (57) | 1233 (49) | |
| Cause of death | .404 | ||||
| Graft rejection | 13 (<1) | 5 (<1) | 14 (<1) | 9 (<1) | |
| Infection | 273 (15) | 94 (17) | 237 (16) | 183 (15) | |
| IPN | 26 (1) | 7 (1) | 22 (1) | 16 (1) | |
| Organ failure | 281 (16) | 73 (13) | 195 (13) | 151 (12) | |
| GVHD | 210 (12) | 73 (13) | 165 (11) | 149 (12) | |
| Recurrent/persistent disease | 758 (42) | 224 (41) | 660 (45) | 569 (46) | |
| Secondary malignancy | 16 (<1) | 8 (1) | 23 (2) | 13 (1) | |
| Hemorrhage | 39 (2) | 8 (1) | 20 (1) | 27 (2) | |
| Other cause | 117 (6) | 35 (6) | 99 (7) | 85 (7) | |
| Unknown | 68 (4) | 20 (4) | 41 (3) | 31 (3) |
IPN, interstitial pneumonia.
Subset analysis focusing on AML patients receiving PBSC grafts
Early CMV reactivation has been previously reported to associate with decreased incidence of relapse in allogeneic HCT patients with AML.9 Specifically, subset analysis was performed on 446 adult patients with AML receiving PBSC grafts following a MA conditioning regimen without serotherapy and who received cyclosporine (CSA) and methotrexate (MTX)-based GVHD prophylaxis. In this subset analysis, CMV reactivation as a time-dependent covariate did not associate with decreased risk for relapse by 1 year (CMV reactivation: n = 113, relapse 26% [95% CI, 18-34] vs no CMV reactivation: n = 333, relapse 27% [95% CI, 22-32%]; P = .80). Likewise, D/R CMV serology itself did not associate with reduced risk for relapse (data not shown).
Discussion
The purpose of this registry study involving 9469 patients from the CIBMTR database was to define the influence of CMV serostatus and reactivation on hematologic disease relapse following HCT using BM or PB grafts. Four disease categories (AML, ALL, MDS, and CML) were analyzed to determine the influence of CMV reactivation and D/R serology on transplant outcomes, including disease relapse, NRM, aGVHD, cGVHD, DFS, and OS. Three main observations were made. First, CMV reactivation resulted in increased NRM and decreased DFS, translating into poorer OS in each disease category. Second, positive CMV serology (any positive D/R vs D−/R−) increased NRM and decreased DFS or OS in transplant patients with AML and ALL, but had no effect in patients with CML or MDS. Third, early CMV reactivation or D/R CMV serology had no effect on the risk of hematologic disease relapse except for transplant patients with ALL in whom positive D/R serology was associated with an increased risk of disease relapse. Overall, CMV reactivation was not associated with attenuation in risk for AML, ALL, CML, or MDS relapse following initial allogeneic HCT.
Lonnqvist et al first reported the potential effects of CMV infection in reducing leukemia relapse following allogeneic HCT.19 Recent studies have also suggested that positive CMV serology or reactivation may prevent leukemia relapse, mostly in patients with AML. For example, positive D/R CMV serology was associated with lower risk for acute leukemia relapse and higher DFS in 140 pediatric patients.8 In 266 adults, patients with early CMV reactivation (prior to D100) experienced decreases in AML relapse (relapse rate in patients with and without CMV reactivation, 9% vs 42%, respectively), but without increases in NRM.9 In 264 adult AML patients, CMV reactivation attenuated leukemia relapse and improved DFS after MA conditioning, but not after RIC.12 Finally, in 2 large database studies involving 76113 and 183620 AML patients, CMV reactivation increased NRM, thereby negating the effect of attenuation in leukemia relapse.
Graft-versus-leukemia activity prevents hematologic malignant disease relapse following allogeneic HCT.21 In this regard, mechanisms for how CMV reactivation might actually protect against AML relapse remain unknown. CMV reactivation may induce expansion in mature natural killer cells (CD56dimNKG2C+CD57+), which have enhanced interferon γ production22 and can mediate a graft-versus-leukemia response against AML.23 In addition, studies have also shown that γδ T cells recognizing CMV peptides are cross-reactive against leukemia cells.24 Consistent with this hypothesis, lymphocyte depletion using CD34 selection and serotherapy can abrogate the benefit of CMV reactivation in preventing hematologic disease relapse.25-27 Ironically, CMV-driven immunity by itself seems not to influence risk in leukemia relapse after HCT, but rather overall immune reconstitution in the allogeneic transplant recipient seems most important.15
The current CIBMTR study performed a subset analysis on AML patients who received MA conditioning and PBSC grafts, as early CMV reactivation in this specific patient population has most often been reported to decrease AML relapse.9 However, results herein do not show a protective effect from either CMV reactivation or positive CMV serology in this patient population. Other single-center studies14,17,25,26 and 3 large dataset multicenter studies18,28 have also not observed a protective effect of CMV serology or reactivation and decreased leukemia relapse. Thus, if an effect of CMV on preventing AML relapse exists, it seems to be limited to transplant center-specific settings, which remain undefined.
The EBMT recently published 2 database studies defining the influence of CMV serostatus and reactivation on allogeneic HCT outcomes. In an analysis involving nearly 17 000 patients with de novo acute leukemia who received BM or PB allografts, Schmidt-Heiber et al observed higher risk for leukemia relapse (decreased leukemia-free survival) in patients with CMV seropositivity in either donor or recipient.18 Ljungman et al analyzed ∼50 000 patients who received allogeneic HCT with BM or PB allografts for malignant and nonmalignant conditions and noted that CMV seronegative patients had higher risk for disease relapse when receiving grafts from CMV seropositive unrelated donors but not from CMV seropositive MSD.28
The current study confirms the continued negative effect that CMV seropositivity and reactivation have on transplant outcomes as also noted in the aforementioned EBMT database studies.18,28 Specifically, positive CMV serostatus increased NRM and lowered DFS in allogeneic transplant patients with ALL and AML, but lowered OS in all disease groups (ALL, AML, CML, and MDS). Likewise, CMV reactivation increased NRM and decreased DFS in all disease groups except CML. Similar to these results, Schmidt-Heiber et al found that any positive D/R CMV serostatus increased 2-year NRM and decreased OS and that CMV reactivation also decreased OS.18 The observed increase in NRM was attributed to death from infection. Interestingly, detrimental effects of positive CMV serostatus were more marked in patients with ALL than those with AML. In our study, for MDS and CML, the discrepancy between CMV reactivation associated with increased NRM, and D/R positive serology not associated with the risk of NRM is intriguing. A possible explanation may be risk factors of NRM that could overwhelm the risk associated to D/R serology and would not be included in our multivariate analysis. For instance, comorbidity index, age of the donor, and time from diagnosis to transplantation are risks factors for NRM among CML29,30 and MDS patients.31,32 In the study of Ljungman et al, positive CMV serostatus was associated with decreased OS in patients receiving MUD grafts but not MSD or mismatched donor allografts.28 Restricting transplant outcome comparisons between D+/R+ and D−/R+, these investigators found that D+ serostatus increased OS and decreased NRM in patients receiving MA conditioning and MUD allografts, but not in patients receiving RIC. Interestingly, D+ serostatus decreased death from infection, particularly viral infection.
Three potential reasons for CMV adversely affecting transplant outcomes include (1) increasing the risk for bacterial and fungal coinfections33 ; (2) increasing organ toxicity directly via CMV infection itself and indirectly via associated side effects of antiviral therapy1 ; and (3) increasing incidence and severity in GVHD.34 In the current CIBMTR study, relapsed disease, GVHD, and organ dysfunction were the top causes of death, consistent with these proposed detrimental effects of CMV. Given these effects, alternative strategies to prevent and to treat CMV reactivation and infection are clearly needed. For example, pharmaceutical agents with better efficacy and reduced toxicity profiles would lessen viral-associated morbidity and mortality.35-37 In this regard, viral-directed adoptive cellular immunotherapy has emerged to advance the therapeutic approach to viral disease in allogeneic HCT38,39 and to decrease the need for conventional pharmaceutical agents with harmful end-organ effects.39,40
This study has several limitations. Inherent to this being a registry study is the incompleteness of the data collected in the CIBMTR database. For example, no data were collected for how CMV reactivation was monitored with respect to tests used and institutional cutoff values applied for implementing preemptive therapy. As a result, inaccurate reporting for CMV reactivation is possible. Similarly, institutional practices for preemptive and prophylactic therapy vary with respect to initiation and duration of antiviral therapy for CMV reactivation, affecting the ability to assess efficacy of initial therapy and duration of CMV reactivation. Last, the study’s retrospective analysis limits generalization of results across transplant settings. Thus, this study finds several differences between groups of disease regarding outcomes that cannot be fully analyzed with the current data. For instance, the observation of better DFS for acute leukemia with a match unrelated compared with a match sibling donor would deserve further study with more extensive data focusing on this issue. Notwithstanding, large multicenter databases like the CIBMTR reduce institutional heterogeneity in clinical practices and enable statistical analyses that cannot be performed at the single institutional level.
Acquiring additional relevant data from participating transplant centers within the CIBMTR would have enhanced statistical analyses in the current study and enabled more meaningful interpretation of results. For example, recording each center’s threshold of CMV reactivation at which time antiviral treatment was initiated, as well as institutional practice guidelines for choice, dose, and duration of antiviral agents used for first-line treatment and prophylaxis, would have been beneficial to this study. Collecting such supplemental data might also be used to establish evidence-based guidelines for CMV prophylaxis and treatment and improve practice consistency among transplant centers. Similarly, implementation of standardized laboratory best practices for CMV testing would improve CMV monitoring across transplant centers. Together, such initiatives would extend our knowledge of CMV infection after HCT, which remains a priority for the transplant community given the virus’ continued negative influence on patient outcomes in the contemporary era.
In conclusion, CMV serostatus and reactivation increase NRM with resultant decreases in DFS and OS following allogeneic HCT. Furthermore, no protective effect of early CMV reactivation in preventing hematologic disease relapse was observed.
Data contained in this manuscript have been previously reported as an oral abstract presentation at the 56th American Society of Hematology Annual Meeting and Exposition, San Francisco, CA, December 6-9, 2014 (Abstract 47).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted on behalf of the Infection and Immune Reconstitution Working Committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) which includes more than 400 members. The CIBMTR is supported by Public Health Service grant/cooperative agreement 5U24-CA076518 from the National Institutes of Health, National Cancer Institute (NCI), National Heart, Lung and Blood Institute (NHLBI), and National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement 5U10HL069294 from NHLBI and NCI; contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc; anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc; Genzyme Corporation; *Gilead Sciences, Inc; Health Research, Inc Roswell Park Cancer Institute; HistoGenetics, Inc; Incyte Corporation; *Jazz Pharmaceuticals, Inc; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc; National Marrow Donor Program; Neovii Biotech NA, Inc; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc; Otsuka America Pharmaceutical, Inc; Otsuka Pharmaceutical Co, Ltd.–Japan; Oxford Immunotec; Perkin Elmer, Inc; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc; Swedish Orphan Biovitrum, Inc; Telomere Diagnostics, Inc; TerumoBCT; Therakos, Inc; University of Minnesota; and *Wellpoint, Inc. *Corporate members.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: M.R., P.T., M. Battiwalla, A.J.B., K.W.A., M.C., J.J.A., C.A.L., M. Boeckh, and M.L.R. conceived and designed the study, collected and assembled the data, and wrote the manuscript; all authors performed data analysis and interpretation of data; and J.S.G., A.S., J.H.A., B.N.S., H.M.L., M.S., W.S., D.M., M.A., M.N., and J.R.W., provided final approval of the manuscript.
Conflict-of-interest disclosure: C.A.L. has consulted for Chimerix. M. Boeckh has consulted for Genentech, Chimerix Inc, Merck, Shire, Clinigen, and Astellas and has received research support from Roche Molecular Systems. All other authors declare no competing financial interests.
Correspondence: Pierre Teira, Hematology Oncology Department, Sainte Justine Hospital, University of Montreal, 3175 Chemin Cote Sainte Catherine, Montreal, QC, Canada H3T 1C5; e-mail: pierre.teira.hsj@ssss.gouv.qc.ca.
References
Author notes
P.T., M. Battiwalla, M.R., and A.J.B. were principal investigators and contributed equally to this work.