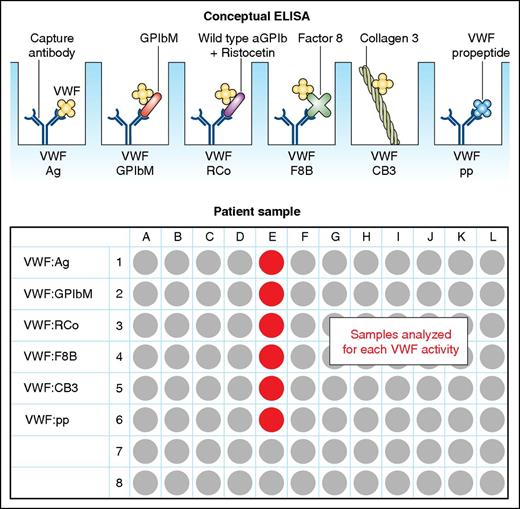
In this issue of Blood, Roberts et al present a novel enzyme-linked immunosorbent assay (ELISA)–based von Willebrand factor (VWF) multiplex activity assay that accurately determines variant von Willebrand disease (VWD) phenotype.1
Graphic of the multiplex ELISA including its multiple assessments of VWF. Ag, antigen; pp, propeptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
Graphic of the multiplex ELISA including its multiple assessments of VWF. Ag, antigen; pp, propeptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
In the 2006 International Society on Thrombosis and Haemostasis classification of VWD, there are 3 recognized types: type 1 VWD is characterized by a partial reduction in VWF and type 3 VWD by its virtual absence. Type 2 VWD is a group of 4 qualitative variants: type 2A, characterized by loss of the most hemostatically active high molecular weight multimers; type 2B, caused by gain-of-function mutations increasing the binding of VWF to platelet glycoprotein Ib (GPIb); type 2M that results from loss-of-function mutations in the platelet binding region of VWF; and type 2N that is characterized by reduced factor VIII (FVIII) binding ability of VWF.2 More recently, type 1C VWD has also been described, which results from increased VWF clearance from the circulation.3 Accurate assignment of VWD type and subtype is not just a form of torture for junior trainees, but can influence treatment decisions and the understanding of inheritance within a family. Assigning VWD phenotype at present requires many specific assays of VWF function that are cumbersome, difficult to standardize, and available only in specialized laboratories. As a result, repeat testing is the norm, and a definitive diagnosis can take weeks or months to achieve, or remain indefinitely elusive. The traditional VWF ristocetin cofactor (VWF:RCo) assay further highlights existing limitations with its high coefficient of variation (up to 50% in some laboratories) and inaccuracy in individuals with the common VWF A1 domain variant, D1472H.4
Therefore, the authors used samples from 160 well-characterized subjects from the Zimmerman Program for the Molecular and Clinical Biology of von Willebrand Disease to develop and validate a new assay. This included 138 VWD patients and 22 individuals with hemophilia A. The multiplex ELISA can measure all of the known hemostatic functions of VWF on a single plate, including VWF binding to platelets (using the VWF:RCo and a non–ristocetin-based mutant GPIb assay, collagen III, and FVIII (VWF:FVIIIB), as well as concentrations of VWF antigen and its propeptide (see figure). The assay had an overall accuracy of 92.5% in the study cohort for the identification of type 1C, 2A, 2B, 2M, and 2N VWD, and the authors predict an 88.1% overall accuracy in the general population. The coefficients of variation for intraplate and interplate testing were impressive at 5.2% and 14.1%, respectively.
Accurate VWD diagnosis has important clinical implications. The misdiagnosis of type 2N VWD as hemophilia A can result in the administration of a coagulation factor concentrate containing FVIII alone, rather than a VWF-containing concentrate and in inaccurate genetic counseling for families. Failure to recognize type 1C VWD or type 2B VWD could result in the inappropriate use of desmopressin with resultant bleeding from rapid VWF clearance or thrombocytopenia.
Despite the technical advantages of this new assay, and its rapid turn around time, questions remain about its widespread implementation. How will laboratories currently offering VWD testing incorporate this assay into their existing diagnostic algorithms? Will it allow smaller, less specialized laboratories to start providing VWD testing? And, if that occurs, will the impressively low coefficient of variation reported in this paper remain?
This assay has the distinct advantage of being based on the widely used ELISA platform and is quick to perform. It shows good correlation with traditional assays currently in use that are more technically challenging and it might offer cost savings by reducing the need for repeat testing. The authors’ conclusion that it should be considered for screening patients requires further consideration and study but conceivably, this assay should improve the future of VWD diagnosis.
Conflict-of-interest disclosure: P.D.J. has received research funding from Bayer, CSL Behring, and Octapharma; and honoraria for educational talks from Baxalta, CSL Behring, and Octapharma.