In this issue of Blood, Flood et al report on bleeding symptoms and laboratory analyses in 482 subjects diagnosed with type 1 von Willebrand disease (VWD) as part of the Zimmerman Program, a multicenter study of VWD.1 Their findings provide insight into the underlying DNA variants in VWD, and also raise questions about the diagnosis of VWD and of mucocutaneous bleeding disorders in general.
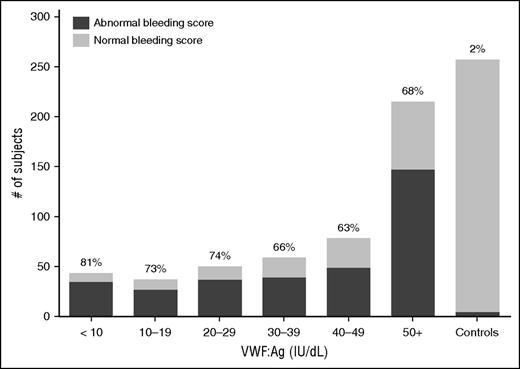
No significant differences in BS for type 1 VWD subjects regardless of VWF:Ag level. This graph shows the number of subjects with abnormal BS (defined as >2 in children <18 years of age, >3 in adult males, and >5 in adult females) in dark gray as compared with those with normal BSs (light gray) for the entire type 1 VWD cohort by VWF:Ag. The percent of each group with abnormal BS is shown at the top of each column. BS was similar tor type 1 subjects regardless of VWF:Ag. See Figure 2 in the article by Flood et al that begins on page 2481.
No significant differences in BS for type 1 VWD subjects regardless of VWF:Ag level. This graph shows the number of subjects with abnormal BS (defined as >2 in children <18 years of age, >3 in adult males, and >5 in adult females) in dark gray as compared with those with normal BSs (light gray) for the entire type 1 VWD cohort by VWF:Ag. The percent of each group with abnormal BS is shown at the top of each column. BS was similar tor type 1 subjects regardless of VWF:Ag. See Figure 2 in the article by Flood et al that begins on page 2481.
VWD is the most common inherited bleeding disorder affecting ∼0.1% of the population.2 Type 1 VWD, accounting for ∼80% of patients diagnosed with VWD, is manifest by a parallel decrease in von Willebrand factor (VWF) protein, VWF activity, and factor VIII activity.3 Patients with type 1 VWD primarily have mucocutaneous bleeding symptoms which include epistaxis, ecchymosis, bleeding with mucosal procedures, and, in females, menorrhagia and postpartum hemorrhage. Prior studies of type 1 VWD patients in Europe (Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD [MCMDM-1VWD] study) and in Canada, demonstrated that many patients diagnosed with type 1 VWD did not have potentially causative DNA variants in the VWF gene.4,5 In both studies, the lower the VWF antigen (VWF:Ag) level, the more likely a DNA variant was found. A potentially causative variant was found in about 50% of subjects with VWF levels of 40 IU/dL.4
In guidelines developed under the auspices of the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) and published in 2008, the recommendation was made to limit the diagnosis of VWD to patients with VWF:Ag or activity levels of 30 IU/dL, and to use the term “low VWF” for patients with levels that are decreased but above 30 IU/dL.6 Concern was voiced about overdiagnosis of patients with mild bleeding symptoms.7 Although the NHLBI guidelines acknowledged that bleeding symptoms in those with low VWF may need treatment, this designation has produced a quandary in diagnosis and treatment of patients with mucocutaneous bleeding symptoms.
A quantitative bleeding assessment tool (BAT) used to calculate a bleeding score (BS) was developed for use in the diagnosis of VWD as part of the MCMDM-1VWD study, and this BAT and modifications thereof have been validated for VWD and other bleeding disorders. In the Zimmerman Program study, the International Society of Thrombosis and Haemostasis BAT was used to quantify bleeding symptoms,8 and results were correlated with plasma and genetic laboratory findings. A striking finding of their study was that the BS did not differ significantly between those diagnosed with VWD, low VWF, or with a historical diagnosis of VWD but normal values at enrollment (see figure).
In the data reported by Flood et al, the likelihood of finding a potentially causative DNA variant was inversely correlated with the VWF:Ag level at low values, with variants found in >90% of subjects with values of <20 IU/dL. However, the percent of subjects with sequence variants found in those with values of 20-29 IU/dL, 30-39 IU/dL, and 40-49 IU/dL were 74%, 58% and 64%, respectively. These differed significantly from that found in subjects with a historical diagnosis of VWD and normal values at enrollment. Variants found in those subjects were similar in number and type to those found in the normal controls. Of note, the VWF coding region is polymorphic and many VWF DNA variants are not associated with VWD.9
What can we learn from the study by Flood et al about patients with type 1 VWD? Many subjects in their study with a historical diagnosis of VWD but with normal values at enrollment most likely have a bleeding disorder other than VWD, given their BS and lack of VWF DNA variants, which contrast to that found in subjects with low VWF levels. Many likely have disorders of platelet function, given the similarity in symptoms to VWD. We do not know if platelet function studies were performed locally, but even if performed, current clinical assays to diagnose platelet function disorders have significant limitations.
Their results provide important new information on type 1 VWD and also raise questions regarding the diagnosis of VWD. The BS and the prevalence of VWF variants were similar in those who would be diagnosed with VWD or “low VWF.” The diagnosis of VWD is complicated by the many factors that affect VWF synthesis, storage granule release, function, and clearance. Many patients with type 1 VWD likely have a complex genetic disorder in which more than one gene coupled with environmental stressors is responsible for their bleeding symptoms. A better understanding of these factors and their impact on hemostasis will lead to improved diagnosis and treatment of bleeding disorders.
Conflict-of-interest disclosure: The author declares no competing financial interests.