Key Points
This study is the first to assess the prognostic value of FVIII-specific antibody data in patients with AHA.
Anti-FVIII IgA, but not immunoglobulin G, autoantibodies at baseline are potential predictors of recurrence and poor outcome of AHA.
Abstract
Neutralizing autoantibodies against factor VIII (FVIII), also called FVIII inhibitors, are the cause of acquired hemophilia A (AHA). They are quantified in the Bethesda assay or Nijmegen-modified Bethesda assay by their ability to neutralize FVIII in normal human plasma. However, FVIII inhibitors do not represent the whole spectrum of anti-FVIII autoantibodies. Here, we studied isotypes, immunoglobulin G subclasses, and apparent affinities of anti-FVIII autoantibodies to assess their prognostic value for the outcome in AHA. We analyzed baseline samples from patients enrolled in the prospective GTH-AH 01/2010 study. Our data suggest that anti-FVIII immunoglobulin A (IgA) autoantibodies are predictors of poor outcome in AHA. Anti-FVIII IgA-positive patients achieved partial remission similar to anti-FVIII IgA-negative patients but had a higher risk of subsequent recurrence. Consequently, IgA-positive patients achieved complete remission less frequently (adjusted hazard ratio [aHR], 0.35; 95% confidence interval [CI], 0.18-0.68; P < .01) and had a higher risk of death (aHR, 2.62; 95% CI, 1.11-6.22; P < .05). Anti-FVIII IgA was the strongest negative predictor of recurrence-free survival after achieving partial remission and remained significant after adjustment for baseline demographic and clinical characteristics. In conclusion, anti-FVIII IgA represents a potential novel biomarker that could be useful to predict prognosis and tailor immunosuppressive treatment of AHA.
Introduction
Acquired hemophilia A (AHA) is a rare and often severe bleeding disorder caused by neutralizing autoantibodies directed against the coagulation factor VIII (FVIII).1 The underlying immune pathogenesis is poorly understood, partly because of the low incidence of AHA, which is estimated to be ∼1.3 cases per million people.2
Major risk factors for the development of AHA include advanced age, diseases such as malignancies and autoimmune disorders, as well as pregnancy and the postpartum period.3,4 Almost half of patients develop autoantibodies spontaneously without an underlying medical condition.1 In clinical practice, neutralizing autoantibodies against FVIII are commonly identified as FVIII inhibitors by using the Bethesda or Nijmegen modified Bethesda Assay (NBA).5 Although these assays have contributed to our understanding of the loss of FVIII function seen in AHA patients, FVIII inhibitors do not reflect the whole picture of FVIII-specific autoimmune responses. Moreover, most FVIII inhibitors in AHA patients have complex reaction kinetics with nonlinear inactivation of FVIII activity, which makes in vitro quantification of FVIII inhibitor titers difficult.6,7 These limitations could explain why the FVIII inhibitor titer analyzed at time of diagnosis is only a weak predictor of bleeding episodes and treatment outcome in AHA patients.7,8 New technologies applied at time of diagnosis are required to obtain a better understanding of predictive immune parameters for treatment outcome in patients with AHA. This can best be studied in a large unselected patient population treated according to a uniform protocol as was applied to the GTH-AH 01/2010 study.9
GTH-AH 01/2010 was a multicenter prospective observational study of 102 patients with AHA who were treated according to a consensus protocol developed by the Acquired Hemophilia Working Group of the German, Austrian and Swiss Thrombosis and Hemostasis Society (GTH).9 Clinical characteristics and widely available laboratory markers were assessed as potential predictors of outcome. FVIII activity at baseline was found as a major predictor of partial remission (PR), complete remission (CR), and overall survival (OS), whereas the local laboratory FVIII inhibitor titer had less impact on clinical outcomes.
A subgroup of this study cohort (81 patients) with sufficient plasma available from the day of study enrollment was included in an immunologic study with the objective to assess the predictive power of FVIII-specific antibody isotypes and immunoglobulin G (IgG) subclasses at baseline for the treatment outcome in patients with AHA. For this purpose, we used a fully validated state-of-the-art enzyme-linked immunosorbent assay (ELISA) platform for the analysis of FVIII-specific immunoglobulin M (IgM), immunoglobulin A (IgA), and IgG subclass (1-4) antibodies as previously described.10 Moreover, all samples that were positive for FVIII-binding antibodies were subjected to the assessment of apparent affinities for the different FVIII-specific antibody populations using a competition-based ELISA platform as described by Hofbauer et al.11
Our data indicate that circulating anti-FVIII IgA autoantibodies, but not anti-FVIII IgG autoantibodies, present at baseline, are potential predictors for a poor recurrence-free survival and a poor overall outcome in patients with AHA.
Methods
Study population
GTH AH 01/2010 was a multicenter prospective observational study of 102 patients with AHA who were treated according to the GTH consensus protocol by 29 registered sites in Austria and Germany.9 The research protocol was approved by the ethics committees of participating institutions. Inclusion and exclusion criteria have been reported before. In brief, patients were eligible if they had AHA (defined as FVIII activity <50 IU/dL and inhibitor ≥0.6 Bethesda units [BU]/mL), gave informed consent, and were enrolled ≤7 days after starting immunosuppressive treatment (IST). The current study was a subgroup analysis of the 81 patients with sufficient citrate plasma from the day of study enrollment available. All patients consented to sample collection and subsequent analysis.
Clinical data and end points
PR, CR, and OS
Baseline characteristics and end points for this subgroup analysis were extracted from the GTH-AH 01/2010 study database. PR, CR, and OS were defined and collected as reported before.9 In brief, PR was defined as FVIII activity restored to >50 IU/dL and no active bleeding after stopping any hemostatic drug for >24 hours. In all patients in this cohort, bleeding and administration of hemostatic drugs had been stopped before FVIII increased to 50 IU/dL, thus only the factor level had practical impact on the time to PR. CR was defined as PR plus negative inhibitor test, prednisolone tapered to <15 mg/d and any other IST stopped. Time to PR and CR was counted from the day of starting IST for AHA. In patients who died before reaching PR or CR, the time to this end point was set to infinity. OS was the time from start of steroid to death or end of study, which was the end of all observations at the day of database closure. The median follow-up period was 283 days (interquartile range, 89-627 days).
Recurrence
Recurrence was defined as a drop of FVIII activity to <50 IU/dL after achieving PR. Early recurrence was a recurrence that occurred after PR but before reaching CR. Late recurrence was recurrence after CR. Recurrence-free survival was defined as the time from PR to any form of recurrence or death.
Ongoing remission
Because the data analysis revealed that early and late recurrence were important issues, we constructed an additional post hoc end point, ongoing remission (OR), defined as complete cessation of any IST while still being in remission (FVIII:C >50% IU/dL). Time to OR was counted from the day of starting IST therapy for AHA.
Immunologic analysis
Detection of FVIII-binding antibodies
Citrated plasma samples were analyzed for FVIII-binding antibodies (differentiated for IgG subclasses 1-4 as well as isotypes IgM and IgA) using fully validated semiquantitative, direct binding ELISA assays, as described by Whelan et al.10 Briefly, the analysis of FVIII-binding antibodies included a multitiered approach composed of screening for positive and negative samples and titer determination. The specificity of FVIII-binding antibodies was confirmed upon assessment of the apparent affinities (see “Assessment of apparent affinities of FVIII-binding antibodies”). During the screening phase, each plasma sample was analyzed twice at a dilution of 1:20. The minimum dilution of 1:20 was chosen to prevent unspecific matrix effects. Cutoffs were established in plasma samples from 85 healthy blood donors, aged 58 to 66 years. If the delta optical densities (DOD) of both analyses were below cutoff, the sample was deemed negative. If the DOD of both analyses were equal to or greater than the cutoff, the sample was considered positive and subsequently analyzed for antibody titers. In case of discrepancy, a third repetition was performed to determine whether the sample to be considered positive or negative. The antibody titer of a sample was defined as the highest dilution that still displayed a positive signal (DOD ≥ cutoff). Samples were diluted in geometric progression, starting at a dilution of 1:20 and continuing in 1:2 dilution steps.
All ELISA assays were validated with regard to within-run (intra)precision, between-run (inter)precision and stability (robustness) to freeze/thaw cycles of samples, as described by Whelan et al.10 The acceptance criterion for precision of all ELISA assays was ±1 titer step. Based on this assay precision, differences in antibody titers between 2 samples had to be at least 3 titer steps to be evaluated as different. Differences of only 1 or 2 titer steps could simply reflect the variability of the assays.
Further details for the detection of FVIII-binding antibodies are provided in supplemental Methods (available on the Blood Web site).
Assessment of apparent affinities of FVIII-binding antibodies
Plasma samples with titers of FVIII-binding antibodies ≥1:80 for any of the IgG subclasses 1-4 or the Ig isotypes IgM or IgA were included in the assessment of apparent affinities for FVIII-binding antibodies. The apparent affinities KA [M−1] of FVIII-binding antibodies, differentiated for IgG subclasses 1-4 and Ig isotypes IgM and IgA, were determined using a competition-based affinity ELISA platform, as described by Hofbauer et al.11 Briefly, the affinity assessment was based on the availability of antibodies for binding to FVIII-coated ELISA plates after competition with FVIII of different molar concentrations in solution. Data for apparent affinities (KA [M−1]) were derived from nonlinear regression modeling of competition ELISA DOD, as described by Stevens et al.12 Antibodies without conclusive apparent affinity were considered unspecific and excluded from analysis. Further details of the test principle of the competition-based affinity ELISA platform are provided in supplemental Methods.
To prove the technical reproducibility of the competition-based affinity ELISA platform, validation experiments were performed involving the assessment of within-run (intra-assay) precision, between-run (interassay) precision and stability (robustness) to freeze/thaw cycles of samples as described by Hofbauer et al.11 The validation of these critical parameters considered the acceptance criteria for ligand binding assays as suggested by the European Medical Agency guideline for bioanalytical method validation.13 The acceptance criterion for all parameters under investigation was a coefficient of variation <25%.
Statistical analysis
Statistical analysis and figure preparation was performed using IBM SPSS Statistics version 23 and GraphPad Prism version 6. Medians and interquartile ranges, or patient/event numbers and frequencies, were used to describe data as appropriate. Correlations were reported using the nonparametric Spearman correlation coefficient. Age at baseline (≤74 years vs >74 years), FVIII activity (<1 IU/dL vs ≥1 IU/dL), FVIII inhibitor concentration (≤20 BU/mL vs >20 BU/mL), and World Health Organization performance status (WHO-PS; ≤2 vs >2) were dichotomized at a clinically useful divider near the median as reported before.9 Frequencies were compared using Fisher’s exact test or χ2 test as indicated. Continuous data were compared among groups by Kruskal-Wallis test. Univariate and multivariate Cox regression analyses were performed for the time from the day of start of steroid therapy to the day of achieving PR, CR, OR, or death (ie, OS). For these analyses, patients were censored at the end of study if they were alive but had not reached the end point. Those who died before reaching PR, CR, or OR were assigned an infinite time to this end point because they could no longer achieve it. Cox regression analysis was also performed for the time from the day of achieving PR to the day of either recurrence or death (ie, recurrence-free survival). For this analysis, patients were censored at the end of study if they were alive and in remission. For all Cox regression models, categorical or categorized baseline variables were entered as independent factors as indicated. Hazard ratios were calculated for each variable together with confidence intervals (CIs). For all analyses, a P value < .05 was considered statistically significant.
Results
Main baseline characteristics and outcome data from the GTH-AH 01/2010 cohort and the subgroup studied here are presented in Table 1. There were no significant differences detected between the 2 sets of patients.
Baseline characteristics and main clinical end points of the entire GTH study cohort and the subgroup of patients with plasma samples available for immunologic analysis
| Characteristic . | GTH-AH 01/2010 population (n = 102) . | Subgroup with sample available (n = 81) . |
|---|---|---|
| Gender, n (%) | ||
| Female | 43 (42) | 35 (43) |
| Male | 59 (58) | 46 (57) |
| Underlying disorder, n (%) | ||
| None/idiopathic | 68 (67) | 53 (65) |
| Autoimmunity | 20 (20) | 14 (17) |
| Malignancy | 13 (13) | 9 (11) |
| Pregnancy | 5 (5) | 5 (6) |
| WHO-PS, n (%) | ||
| 0 | 15 (15) | 13 (16) |
| 1 | 26 (25) | 19 (23) |
| 2 | 23 (23) | 18 (22) |
| 3 | 22 (22) | 16 (20) |
| 4 | 15 (15) | 15 (19) |
| 5 | 1 (1) | 0 (0) |
| Age (y), median (IQR) | 74 (61-81) | 74 (62-81) |
| FVIII activity (IU/dL), median (IQR) | 1.4 (<1-3.9) | 1.0 (<1-3.0) |
| FVIII inhibitor concentration (BU/mL), median (IQR) | 19 (7.5-71) | 19 (7.5-71) |
| Partial remission | ||
| Achieved, n (%) | 85 (83) | 70 (86) |
| Time (days), median (IQR) | 30 (19-51) | 30 (18-50) |
| Complete remission | ||
| Achieved, n (%) | 62 (61) | 52 (65) |
| Time (days), median (IQR) | 69 (48-102) | 69 (50-98) |
| Characteristic . | GTH-AH 01/2010 population (n = 102) . | Subgroup with sample available (n = 81) . |
|---|---|---|
| Gender, n (%) | ||
| Female | 43 (42) | 35 (43) |
| Male | 59 (58) | 46 (57) |
| Underlying disorder, n (%) | ||
| None/idiopathic | 68 (67) | 53 (65) |
| Autoimmunity | 20 (20) | 14 (17) |
| Malignancy | 13 (13) | 9 (11) |
| Pregnancy | 5 (5) | 5 (6) |
| WHO-PS, n (%) | ||
| 0 | 15 (15) | 13 (16) |
| 1 | 26 (25) | 19 (23) |
| 2 | 23 (23) | 18 (22) |
| 3 | 22 (22) | 16 (20) |
| 4 | 15 (15) | 15 (19) |
| 5 | 1 (1) | 0 (0) |
| Age (y), median (IQR) | 74 (61-81) | 74 (62-81) |
| FVIII activity (IU/dL), median (IQR) | 1.4 (<1-3.9) | 1.0 (<1-3.0) |
| FVIII inhibitor concentration (BU/mL), median (IQR) | 19 (7.5-71) | 19 (7.5-71) |
| Partial remission | ||
| Achieved, n (%) | 85 (83) | 70 (86) |
| Time (days), median (IQR) | 30 (19-51) | 30 (18-50) |
| Complete remission | ||
| Achieved, n (%) | 62 (61) | 52 (65) |
| Time (days), median (IQR) | 69 (48-102) | 69 (50-98) |
IQR, interquartile range.
Anti-FVIII Ig isotypes, subclasses, and apparent affinity
All patients had anti-FVIII antibodies of at least 1 Ig isotype detectable. The most prevalent IgG subclasses were IgG4 and IgG1 (98% and 88% of patients, respectively; Table 2). IgG4 and IgG1 also presented the highest antibody titers (median 1:5120 and 1:640, respectively) and apparent affinities (median KA 5.8 × 1010 M−1 and 1.4 × 1010 M−1, respectively). IgG2, IgG3, and IgA were detected in 77%, 41%, and 37% of patients, respectively, and had lower titers (median 1:80) and apparent affinities (median KA 1.9 × 109 M−1, 1.3 × 1010 M−1, 1.7 × 109 M−1, respectively; Table 2). IgM was infrequently detected (9%) at low titers (median 1:80).
Ig isotype and IgG subclass distribution, titer, and affinity of anti-FVIII antibodies in 81 patients with AHA
| Isotype or subclass . | Positive screening, n (%) . | Titer in positive patients, median (IQR) . | Apparent affinity (main cluster) . | Apparent affinity (second cluster, if detected) . | ||
|---|---|---|---|---|---|---|
| n . | KA [M−1] − median (IQR) . | n . | KA [M−1] − median (IQR) . | |||
| IgG1 | 71 (88) | 1:640 (1:320-1:2560) | 70 | 1.4 × 1010 (0.8 × 1010-4.2 × 1010) | 15 | 7.5×107 (4.5×107-9.4×107) |
| IgG2 | 62 (77) | 1:80 (1:40-1:320) | 40 | 1.9 × 109 (1.0 × 109-3.2 × 109) | 2 | 5.7×107 (4.8×107-6.6×107) |
| IgG3 | 33 (41) | 1:80 (1:40-1:320) | 19 | 1.3 × 1010 (0.5 × 1010-1.8 × 1010) | 5 | 9.7×107 (6.8×107-9.9×107) |
| IgG4 | 79 (98) | 1:5120 (1:1280-1:20 480) | 77 | 5.8 × 1010 (2.4 × 1010-1.3 × 1011) | 6 | 3.8×109 (2.9×109-5.2×109) |
| IgA | 37 (46) | 1:80 (1:40-1:160) | 18 | 1.7 × 109 (0.9 × 109-4.6 × 109) | 8 | 5.4×107 (4.6×107-5.5×107) |
| IgM | 7 (9) | 1:80 (1:40-1:80) | 0 | n/d | 0 | n/d |
| Isotype or subclass . | Positive screening, n (%) . | Titer in positive patients, median (IQR) . | Apparent affinity (main cluster) . | Apparent affinity (second cluster, if detected) . | ||
|---|---|---|---|---|---|---|
| n . | KA [M−1] − median (IQR) . | n . | KA [M−1] − median (IQR) . | |||
| IgG1 | 71 (88) | 1:640 (1:320-1:2560) | 70 | 1.4 × 1010 (0.8 × 1010-4.2 × 1010) | 15 | 7.5×107 (4.5×107-9.4×107) |
| IgG2 | 62 (77) | 1:80 (1:40-1:320) | 40 | 1.9 × 109 (1.0 × 109-3.2 × 109) | 2 | 5.7×107 (4.8×107-6.6×107) |
| IgG3 | 33 (41) | 1:80 (1:40-1:320) | 19 | 1.3 × 1010 (0.5 × 1010-1.8 × 1010) | 5 | 9.7×107 (6.8×107-9.9×107) |
| IgG4 | 79 (98) | 1:5120 (1:1280-1:20 480) | 77 | 5.8 × 1010 (2.4 × 1010-1.3 × 1011) | 6 | 3.8×109 (2.9×109-5.2×109) |
| IgA | 37 (46) | 1:80 (1:40-1:160) | 18 | 1.7 × 109 (0.9 × 109-4.6 × 109) | 8 | 5.4×107 (4.6×107-5.5×107) |
| IgM | 7 (9) | 1:80 (1:40-1:80) | 0 | n/d | 0 | n/d |
Screening was done twice at a dilution of 1:20 and considered positive (positive screening) if at least one test yielded a differential optical density ≥ cutoff. Samples with positive screening were subjected to titer titration, starting at a dilution of 1:20 and continuing in 1:2 dilution steps (titer in positive patients). Only samples with titers ≥1:80 were subject to assessment of apparent affinity. If samples exhibited a bimodal affinity distribution, the lower KA was assigned to a second antibody cluster
IQR, interquartile range; KA, affinity constant; n/d, not determined.
Some samples exhibited a bimodal affinity distribution indicating 2 different clusters of antibodies. Consistent with previous data,11 the apparent affinity KA of the second antibody cluster was 10- to 1000-fold lower than that of the high-affinity population (Table 2). The frequency of detection of a second, low-affinity cluster varied according to isotype or subclass (IgG1, 21%; IgG2, 5%; IgG3, 26%; IgG4, 8%; and IgA, 44%).
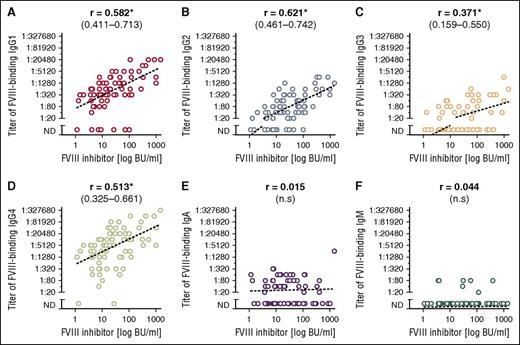
The anti-FVIII antibody titer of all IgG subclasses correlated with the NBA FVIII inhibitor titer, whereas the IgA and IgM titers did not (Figure 1). No correlation was observed between apparent antibody affinity and FVIII inhibitor titer or residual FVIII activity (data not shown).
Correlation between titers of FVIII-binding antibodies and FVIII inhibitor titers. (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgA, and (F) IgM. Spearman correlation coefficients with 95% CIs are given above the panels. Statistically significant correlations are marked with an asterisk (P < .05). ND, not detectable.
Correlation between titers of FVIII-binding antibodies and FVIII inhibitor titers. (A) IgG1, (B) IgG2, (C) IgG3, (D) IgG4, (E) IgA, and (F) IgM. Spearman correlation coefficients with 95% CIs are given above the panels. Statistically significant correlations are marked with an asterisk (P < .05). ND, not detectable.
Presence or absence of anti-FVIII Ig isotypes/IgG subclasses and clinical outcome
We first analyzed the effect of positive screening results for anti-FVIII immunoglobulin isotypes and IgG subclasses on established end points of the GTH-AH 01/2010 study using Cox regression modeling. There was a significant effect of anti-FVIII IgA on CR and OS that remained significant after correction for age, gender, underlying disorder, WHO-PS, baseline FVIII activity, and FVIII inhibitor titer (Table 3). IgA-positive patients achieved CR less often and later (CR in 16 of 37 [43%], after a median of 78 days) as compared with IgA-negative patients (CR in 36 of 44 [82%], after a median of 68 days). This was expressed by an adjusted hazard ratio (aHR) of 0.35 (95% CI, 0.18-0.68), indicating that the likelihood of CR was ∼3 times lower in IgA-positive patients (Table 3). Only ∼50% of IgA-positive patients (18 of 37) were alive at the end of study, whereas 80% of IgA-negative patients (35 of 44) were alive. The Kaplan Meier estimated 1-year survival rates for IgA-positive and negative patients were 52% and 81%, respectively. An aHR of 2.62 (95% CI, 1.11-6.22) indicated that IgA-positive patients had a 2- to 3-fold higher risk of death (Table 3). The most frequent cause of death was infection in the entire patient cohort, regardless of the antibody characteristics. The crude risk of dying from infection was 37% in IgA-positive patients vs 9% in IgA-negative patients (P < .05). The risk of death from other causes was not significantly different among IgA-positive and negative patients (supplemental Table 1).
Presence or absence of anti-FVIII immunoglobulin isotypes and IgG subclasses as predictors of remission and survival in 81 patients with AHA
| Variable . | Partial remission . | Complete remission . | Overall survival . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | |
| IgG1 positive (n = 71) | 0.54 (0.27-1.07) | — | 1.02 (0.44-2.40) | — | 0.76 (0.26-2.20) | — |
| IgG2 positive (n = 62) | 0.53 (0.31-0.92)* | 0.56 (0.31-1.03) | 0.85 (0.45-1.63) | — | 0.97 (0.39-2.40) | — |
| IgG3 positive (n = 33) | 1.09 (0.68-1.75) | — | 0.85 (0.49-1.49) | — | 0.85 (0.40-1.81) | — |
| IgG4 positive (n = 79) | 0.55 (0.13-2.27) | — | 0.30 (0.07-1.25) | — | 21 (0.001-∞) | — |
| IgA positive (n = 37) | 0.63 (0.39-1.02) | — | 0.34 (0.19-0.61)*** | 0.35 (0.18-0.68)** | 3.46 (1.54-7.75)** | 2.62 (1.11-6.22)* |
| IgM positive (n = 7) | 1.06 (0.48-2.31) | — | 0.86 (0.34-2.17) | — | 0.62 (0.15-2.63) | — |
| Variable . | Partial remission . | Complete remission . | Overall survival . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | |
| IgG1 positive (n = 71) | 0.54 (0.27-1.07) | — | 1.02 (0.44-2.40) | — | 0.76 (0.26-2.20) | — |
| IgG2 positive (n = 62) | 0.53 (0.31-0.92)* | 0.56 (0.31-1.03) | 0.85 (0.45-1.63) | — | 0.97 (0.39-2.40) | — |
| IgG3 positive (n = 33) | 1.09 (0.68-1.75) | — | 0.85 (0.49-1.49) | — | 0.85 (0.40-1.81) | — |
| IgG4 positive (n = 79) | 0.55 (0.13-2.27) | — | 0.30 (0.07-1.25) | — | 21 (0.001-∞) | — |
| IgA positive (n = 37) | 0.63 (0.39-1.02) | — | 0.34 (0.19-0.61)*** | 0.35 (0.18-0.68)** | 3.46 (1.54-7.75)** | 2.62 (1.11-6.22)* |
| IgM positive (n = 7) | 1.06 (0.48-2.31) | — | 0.86 (0.34-2.17) | — | 0.62 (0.15-2.63) | — |
Hazard ratios (HRs) from Cox regression models with time to end point as dependent variable and presence/absence of immunoglobulin isotype or subclass as independent variable. aHRs were adjusted for age group (≤ or >74 y), gender (female or male), underlying disorder (malignancy, autoimmunity, pregnancy present or not), baseline FVIII:C (< or ≥1 IU/dL), NBA inhibitor titer (≤ or >20 BU/mL), and WHO-PS (≤ or >2) as categorical variables. Adjustment was not done for isotypes/subclasses that were not significant in univariate analysis. An HR/aHR < 1 denotes a slower rate in achieving the end point; an HR/aHR > 1 indicates a faster rate in achieving the end point.
HR, hazard ratio; —, not applicable because of insignificant result in univariate analysis.
P < .05, **P < .01, ***P < .001.
Figure 2 illustrates the relative impact of anti-FVIII IgA and other prognostic factors previously established in the GTH-AH 01/2010 study in a multivariate Cox regression model. Low baseline FVIII (<1 IU/dL), high FVIII inhibitor titer (>20 BU/mL), poor WHO-PS (>2), and detectable anti-FVIII IgA were independent predictors of a poor chance of CR. Malignancy, poor WHO-PS, and detectable anti-FVIII IgA were independent predictors of poor OS. Of note, the significant association of low baseline FVIII with poor OS, as found in the GTH-AH 01/2010 study, disappeared when IgA was introduced into the multivariate model.
Effects of anti-FVIII IgA and other baseline characteristics on CR (left) and OS (right).
Effects of anti-FVIII IgA and other baseline characteristics on CR (left) and OS (right).
Titers of anti-FVIII Ig isotypes/IgG subclasses and clinical outcome
There was a trend toward lower likelihood of PR and CR with high autoantibody titers for most IgG subclasses. These trends, however, were either not statistically significant or disappeared after multivariate adjustment. In contrast, IgA titers were significantly associated with CR and OS independent of other baseline characteristics (Table 4).
Titer of anti-FVIII Ig isotypes and IgG subclasses as predictors of remission and survival in 81 patients with AHA
| Variable . | Partial remission . | Complete remission . | Overall survival . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | |
| IgG1 | ||||||
| Negative (n = 10) | 1 | 1 | 1 | — | 1 | — |
| ≤ median (≤1:640, n = 35) | 0.71 (0.34-1.46) | 0.82 (0.37-1.81) | 1.31 (0.54-3.20) | — | 0.58 (0.18-1.88) | — |
| > median (> 1:640, n = 36) | 0.44 (0.21-0.90)* | 0.62 (0.27-1.41) | 0.81 (0.33-2.02) | — | 0.94 (0.31-2.85) | — |
| IgG2 | — | — | ||||
| Negative (n = 19) | 1 | 1 | 1 | — | 1 | — |
| ≤ median (≤1:80, n = 33) | 0.61 (0.33-1.12) | 0.59 (0.31-1.12) | 1.03 (0.51-2.08) | — | 0.90 (0.33-2.43) | — |
| > median (>1:80, n = 29) | 0.46 (0.25-0.86)* | 0.52 (0.25-1.05) | 0.69 (0.33-1.45) | — | 1.06 (0.39-2.86) | — |
| IgG3 | — | — | ||||
| Negative (n = 48) | 1 | — | 1 | — | 1 | — |
| ≤ median (≤1:80, n=17) | 1.48 (0.83-2.65) | — | 1.47 (0.78-2.76) | — | 0.69 (0.25-1.87) | — |
| > median (>1:80, n=16) | 0.84 (0.46-1.55) | — | 0.43 (0.18-1.03) | — | 1.05 (0.41-2.67) | — |
| IgG4 | — | — | ||||
| Negative (n = 2) | 1 | — | 1 | — | 1 | — |
| ≤ median (≤1:5120, n = 44) | 0.69 (0.16-2.87) | — | 0.30 (0.07-1.29) | — | 10 607 (0-∞) | — |
| > median (>1:5120, n = 35) | 0.43 (0.10-1.84) | — | 0.30 (0.07-1.28) | — | 6,644 (0-∞) | — |
| IgA | — | |||||
| Negative (n = 44) | 1 | — | 1 | 1 | 1 | 1 |
| ≤ median (≤1:80, n = 24) | 0.66 (0.38-1.15) | — | 0.47 (0.24-0.89)* | 0.46 (0.23-0.93)* | 2.42 (0.95-6.17) | 2.27 (0.82-6.24) |
| > median (> 1:80, n = 13) | 0.57 (0.29-1.15) | — | 0.15 (0.05-0.50)** | 0.15 (0.04-0.55)** | 5.76 (2.30-14.5)*** | 3.20 (1.05-9.73)* |
| Variable . | Partial remission . | Complete remission . | Overall survival . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | HR (95% CI) . | aHR (95% CI) . | |
| IgG1 | ||||||
| Negative (n = 10) | 1 | 1 | 1 | — | 1 | — |
| ≤ median (≤1:640, n = 35) | 0.71 (0.34-1.46) | 0.82 (0.37-1.81) | 1.31 (0.54-3.20) | — | 0.58 (0.18-1.88) | — |
| > median (> 1:640, n = 36) | 0.44 (0.21-0.90)* | 0.62 (0.27-1.41) | 0.81 (0.33-2.02) | — | 0.94 (0.31-2.85) | — |
| IgG2 | — | — | ||||
| Negative (n = 19) | 1 | 1 | 1 | — | 1 | — |
| ≤ median (≤1:80, n = 33) | 0.61 (0.33-1.12) | 0.59 (0.31-1.12) | 1.03 (0.51-2.08) | — | 0.90 (0.33-2.43) | — |
| > median (>1:80, n = 29) | 0.46 (0.25-0.86)* | 0.52 (0.25-1.05) | 0.69 (0.33-1.45) | — | 1.06 (0.39-2.86) | — |
| IgG3 | — | — | ||||
| Negative (n = 48) | 1 | — | 1 | — | 1 | — |
| ≤ median (≤1:80, n=17) | 1.48 (0.83-2.65) | — | 1.47 (0.78-2.76) | — | 0.69 (0.25-1.87) | — |
| > median (>1:80, n=16) | 0.84 (0.46-1.55) | — | 0.43 (0.18-1.03) | — | 1.05 (0.41-2.67) | — |
| IgG4 | — | — | ||||
| Negative (n = 2) | 1 | — | 1 | — | 1 | — |
| ≤ median (≤1:5120, n = 44) | 0.69 (0.16-2.87) | — | 0.30 (0.07-1.29) | — | 10 607 (0-∞) | — |
| > median (>1:5120, n = 35) | 0.43 (0.10-1.84) | — | 0.30 (0.07-1.28) | — | 6,644 (0-∞) | — |
| IgA | — | |||||
| Negative (n = 44) | 1 | — | 1 | 1 | 1 | 1 |
| ≤ median (≤1:80, n = 24) | 0.66 (0.38-1.15) | — | 0.47 (0.24-0.89)* | 0.46 (0.23-0.93)* | 2.42 (0.95-6.17) | 2.27 (0.82-6.24) |
| > median (> 1:80, n = 13) | 0.57 (0.29-1.15) | — | 0.15 (0.05-0.50)** | 0.15 (0.04-0.55)** | 5.76 (2.30-14.5)*** | 3.20 (1.05-9.73)* |
Hazard ratios from Cox regression models with time to end point as dependent variable and presence/absence of immunoglobulin isotype or IgG subclass as independent variable. aHRs were adjusted for baseline variables as indicated in Table 3.
HR, hazard ratio; —, not applicable because of insignificant result in univariate analysis.
*P < .05, **P < .01, ***P < .001.
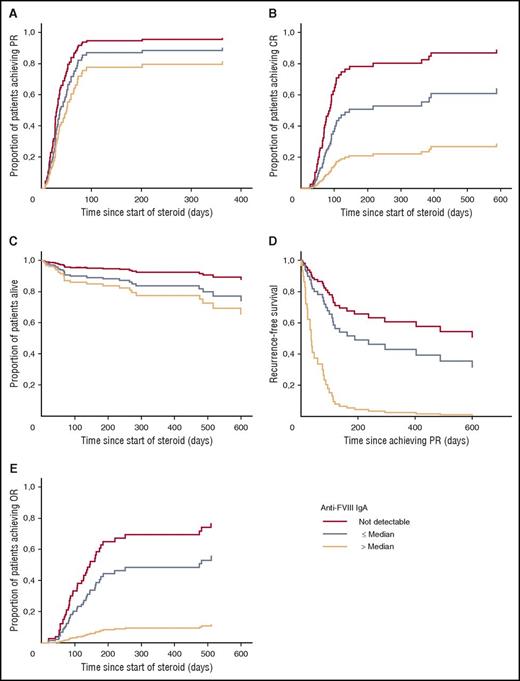
Data from the Cox regression models are depicted in Figure 3. IgA did not have an effect on PR (Figure 3A). However, the likelihood of achieving CR decreased from IgA negative (aHR 1) over IgA titers ≤ median (aHR, 0.47; P < .05) to IgA titers > median (aHR, 0.15; P < .01) (Figure 3B). The risk of death increased from IgA negative (aHR, 1) over IgA titers ≤ median (aHR, 2.27; not significant) to IgA titers > median (aHR, 3.20; P < .05) (Figure 3C).
Effect of anti-FVIII IgA on clinical end points in AHA. Cox proportional hazard model of the time to end point with anti-FVIII IgA (negative, titer ≤ median, titer > median), age, gender, underlying disorder, WHO-PS, FVIII inhibitor concentration, and baseline FVIII as categorical covariates. P values are for difference between IgA-negative vs IgA-positive titer > median. (A) Partial remission (time since start of steroid, not significant). (B) CR (time since start of steroid, P < .01). (C) OS (time since start of steroid, P < .05). (D) Recurrence-free survival (time since achieving partial remission, P < .05). (E) OR after full stop of immunosuppressive treatment (times since start of steroid, P < .05).
Effect of anti-FVIII IgA on clinical end points in AHA. Cox proportional hazard model of the time to end point with anti-FVIII IgA (negative, titer ≤ median, titer > median), age, gender, underlying disorder, WHO-PS, FVIII inhibitor concentration, and baseline FVIII as categorical covariates. P values are for difference between IgA-negative vs IgA-positive titer > median. (A) Partial remission (time since start of steroid, not significant). (B) CR (time since start of steroid, P < .01). (C) OS (time since start of steroid, P < .05). (D) Recurrence-free survival (time since achieving partial remission, P < .05). (E) OR after full stop of immunosuppressive treatment (times since start of steroid, P < .05).
Apparent affinity of anti-FVIII Immunoglobulin and clinical outcome
For all anti-FVIII IgG subclasses and for anti-FVIII IgA, we compared patients, who were negative vs low-affinity antibody positive (KA ≤ median) vs high-affinity antibody positive (KA > median). We did not observe any association with PR, CR, or OS (data not shown). Patients with a secondary, low-affinity IgG1 antibody cluster (15 of 81 patients) tended to have a higher likelihood of PR (dual vs single IgG1 population: aHR, 1.99; 95% CI, 1.02-3.92; P < .05) and CR (aHR, 2.25; 95% CI, 1.03-4.92; P < .05). Otherwise no impact of apparent affinity on outcomes was detected.
Impact of anti-FVIII IgA
We found that anti-FVIII IgA was associated with a reduced likelihood of CR and poor OS, but not with PR (see above). To better understand the impact of IgA on these clinical outcomes, we compared baseline characteristics, treatment, and more detailed outcome information between patients with negative IgA, IgA titers ≤ median, and IgA titers > median (Table 5). Patients with anti-FVIII IgA titers > median were more often male than patients with IgA titers ≤ median or absent anti-FVIII IgA. Age, underlying disorders, baseline FVIII activity, FVIII inhibitor titer, and treatment were not different according to anti-FVIII IgA status.
Selected patient characteristics and outcomes according to anti-FVIII IgA titer
| Characteristic . | IgA negative (n = 44) . | IgA ≤ median (≤1:80) (n = 24) . | IgA > median (>1:80) (n = 13) . | P for difference . |
|---|---|---|---|---|
| Gender, n (%) | .0026 | |||
| Female | 22 (50) | 13 (54) | 0 (0) | |
| Male | 22 (50) | 11 (46) | 13 (100) | |
| Underlying disorder, n (%) | .6211 | |||
| None/idiopathic | 25 (57) | 17 (71) | 11 (85) | |
| Autoimmunity | 9 (21) | 4 (17) | 1 (8) | |
| Malignancy | 6 (13) | 2 (8) | 1 (8) | |
| Pregnancy | 4 (9) | 1 (4) | 0 (0) | |
| Age (y), median (IQR) | 74 (61-81) | 76 (71-83) | 74 (61-78) | .8623 |
| Factor VIII activity (IU/dL), median (IQR) | 1.0 (<1-3.7) | <1 (<1-2.4) | 2.0 (<1-3.6) | .8073 |
| Inhibitor concentration (BU/mL), median (IQR) | 19 (7.4-73) | 19 (7.4-67) | 24 (9.4-114) | .8632 |
| Treatment received, n (%) | ||||
| Steroid | 44 (100) | 24 (100) | 13 (100) | — |
| Cyclophosphamide | 15 (34) | 11 (46) | 5 (38) | .6355 |
| Rituximab | 12 (27) | 5 (21) | 6 (46) | .2568 |
| Outcomes, n (%) | ||||
| PR achieved | 41 (93) | 19 (79) | 10 (77) | .1504 |
| CR achieved | 36 (82) | 13 (54) | 3 (23) | .0003 |
| OR achieved | 31 (70) | 13 (54) | 1 (8) | .0003 |
| Early recurrence | 5 (12) | 4 (21) | 5 (50) | .0273 |
| Late recurrence | 7 (19) | 3 (23) | 1 (33) | .8358 |
| Characteristic . | IgA negative (n = 44) . | IgA ≤ median (≤1:80) (n = 24) . | IgA > median (>1:80) (n = 13) . | P for difference . |
|---|---|---|---|---|
| Gender, n (%) | .0026 | |||
| Female | 22 (50) | 13 (54) | 0 (0) | |
| Male | 22 (50) | 11 (46) | 13 (100) | |
| Underlying disorder, n (%) | .6211 | |||
| None/idiopathic | 25 (57) | 17 (71) | 11 (85) | |
| Autoimmunity | 9 (21) | 4 (17) | 1 (8) | |
| Malignancy | 6 (13) | 2 (8) | 1 (8) | |
| Pregnancy | 4 (9) | 1 (4) | 0 (0) | |
| Age (y), median (IQR) | 74 (61-81) | 76 (71-83) | 74 (61-78) | .8623 |
| Factor VIII activity (IU/dL), median (IQR) | 1.0 (<1-3.7) | <1 (<1-2.4) | 2.0 (<1-3.6) | .8073 |
| Inhibitor concentration (BU/mL), median (IQR) | 19 (7.4-73) | 19 (7.4-67) | 24 (9.4-114) | .8632 |
| Treatment received, n (%) | ||||
| Steroid | 44 (100) | 24 (100) | 13 (100) | — |
| Cyclophosphamide | 15 (34) | 11 (46) | 5 (38) | .6355 |
| Rituximab | 12 (27) | 5 (21) | 6 (46) | .2568 |
| Outcomes, n (%) | ||||
| PR achieved | 41 (93) | 19 (79) | 10 (77) | .1504 |
| CR achieved | 36 (82) | 13 (54) | 3 (23) | .0003 |
| OR achieved | 31 (70) | 13 (54) | 1 (8) | .0003 |
| Early recurrence | 5 (12) | 4 (21) | 5 (50) | .0273 |
| Late recurrence | 7 (19) | 3 (23) | 1 (33) | .8358 |
P values indicate statistical significance from χ2 test or Kruskal-Wallis test as appropriate.
IQR, interquartile range.
Table 5 further shows that PR was not different with regard to anti-FVIII IgA status, whereas achieving CR was much less likely in patients with IgA titers > median (23%) vs IgA titers ≤ median (54%) or absent IgA (82%). This was attributable to a higher risk of early recurrence (ie, recurrence within the 6-week steroid tapering phase after achieving PR and before achieving CR). Thus, 5 of 41 (12%) IgA-negative patients but 4 of 19 (21%) patients with IgA titers ≤ median and 5 of 10 (50%) patients with IgA titers > median experienced early recurrence (P < .05).
Because the GTH treatment protocol recommended increasing the steroid dose to the last effective dose in case of recurrence, we observed a longer exposure to steroids in recurring patients. An additional post hoc end point, OR after complete cessation of any IST, was constructed and was reached by 31 of 44 (70%) IgA-negative patients, 13 of 24 (54%) patients with IgA ≤ median but only 1 of 13 (8%) patients with IgA titers > median (Table 5). This finding was confirmed in the Cox regression model after adjustment for age, gender, underlying disorder, WHO-PS, baseline FVIII activity, and FVIII inhibitor titer (Figure 3E); patients without IgA (aHR 1), or IgA ≤ median (aHR, 0.56; 95% CI, 0.28-1.11) had a better chance of achieving OR than patients with IgA > median (aHR, 0.08; 95% CI, 0.01-0.67).
Recurrence-free survival was further analyzed using Cox regression analysis in the 70 patients who had achieved PR. We found that anti-FVIII IgA-positive patients had higher risk of recurrence or death as compared with IgA-negative patients (Table 6). Other baseline characteristics had little or no impact on recurrence-free survival. After adjustment for age group, gender, underlying disorder, and WHO-PS, positive anti-FVIII IgA was still associated with a poor recurrence-free survival (aHR, 2.61; 95% CI, 1.20-5.70; P = .016). This effect was again much stronger for patients with anti-FVIII IgA titers > median (aHR, 7.51; 95% CI, 2.45-23.0; P < .0001). Figure 3D illustrates recurrence-free survival according to anti-FVIII IgA status adjusted for other baseline parameters.
Recurrence and mortality after achieving PR: univariate analysis of anti-FVIII IgA and other baseline characteristics
| Baseline variable . | Recurrence, n (%) . | Death, n (%) . | Recurrence-free survival (days), median (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Anti-FVIII IgA | |||||
| Positive (n = 29) | 13 (45) | 11 (38) | 81 (53-109) | 2.17 (1.13-4.20) | .021 |
| Negative (n = 41) | 12 (29) | 6 (15) | Not reached | ||
| Factor VIII activity | |||||
| <1 IU/dL (n = 31) | 10 (26) | 9 (23) | 113 (0-316) | 1.75 (0.92-3.35) | .089 |
| ≥1 IU/dL (n = 39) | 15 (48) | 8 (26) | Not reached | ||
| FVIII inhibitor | |||||
| ≤20 BU/mL (n = 39) | 15 (38) | 9 (23) | 119 (83-155) | 1.30 (0.67-2.52) | .431 |
| >20 BU/mL (n = 31) | 10 (32) | 8 (26) | Not reached | ||
| Gender | |||||
| Female (n = 33) | 9 (27) | 5 (15) | Not reached | 0.54 (0.28-1.04) | .067 |
| Male (n = 37) | 16 (43) | 12 (32) | 112 (85-139) | ||
| Age | |||||
| ≤74 y (n = 40) | 15 (38) | 10 (25) | 404 (0-923) | 0.86 (0.45-1.66) | .652 |
| >74 y (n = 30) | 10 (33) | 7 (23) | 135 (0-315) | ||
| WHO-PS | |||||
| Good (≤ 2, n = 45) | 18 (40) | 5 (11) | Not reached | 0.55 (0.28-1.05) | .071 |
| Poor (> 2, n = 25) | 7 (28) | 12 (48) | 93 (0-203) | ||
| Underlying disorder | |||||
| Autoimmunity (n = 13) | 6 (46) | 3 (23) | 78 (0-220) | 1.77 (0.84-3.76) | .136 |
| Malignancy (n = 6) | 3 (50) | 3 (50) | 109 (0-235) | 2.19 (0.84-5.68) | .109 |
| Pregnancy (n = 5) | 2 (40) | 0 (0) | Not reached | 0.75 (0.18-3.12) | .691 |
| Baseline variable . | Recurrence, n (%) . | Death, n (%) . | Recurrence-free survival (days), median (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|
| Anti-FVIII IgA | |||||
| Positive (n = 29) | 13 (45) | 11 (38) | 81 (53-109) | 2.17 (1.13-4.20) | .021 |
| Negative (n = 41) | 12 (29) | 6 (15) | Not reached | ||
| Factor VIII activity | |||||
| <1 IU/dL (n = 31) | 10 (26) | 9 (23) | 113 (0-316) | 1.75 (0.92-3.35) | .089 |
| ≥1 IU/dL (n = 39) | 15 (48) | 8 (26) | Not reached | ||
| FVIII inhibitor | |||||
| ≤20 BU/mL (n = 39) | 15 (38) | 9 (23) | 119 (83-155) | 1.30 (0.67-2.52) | .431 |
| >20 BU/mL (n = 31) | 10 (32) | 8 (26) | Not reached | ||
| Gender | |||||
| Female (n = 33) | 9 (27) | 5 (15) | Not reached | 0.54 (0.28-1.04) | .067 |
| Male (n = 37) | 16 (43) | 12 (32) | 112 (85-139) | ||
| Age | |||||
| ≤74 y (n = 40) | 15 (38) | 10 (25) | 404 (0-923) | 0.86 (0.45-1.66) | .652 |
| >74 y (n = 30) | 10 (33) | 7 (23) | 135 (0-315) | ||
| WHO-PS | |||||
| Good (≤ 2, n = 45) | 18 (40) | 5 (11) | Not reached | 0.55 (0.28-1.05) | .071 |
| Poor (> 2, n = 25) | 7 (28) | 12 (48) | 93 (0-203) | ||
| Underlying disorder | |||||
| Autoimmunity (n = 13) | 6 (46) | 3 (23) | 78 (0-220) | 1.77 (0.84-3.76) | .136 |
| Malignancy (n = 6) | 3 (50) | 3 (50) | 109 (0-235) | 2.19 (0.84-5.68) | .109 |
| Pregnancy (n = 5) | 2 (40) | 0 (0) | Not reached | 0.75 (0.18-3.12) | .691 |
Hazard ratios and P values are from univariate log-rank tests for recurrence-free survival. See “Results” for information on multivariate analysis.
HR, hazard ratio.
Discussion
This study is the first to assess the predictive power of FVIII-specific immunologic baseline data for the treatment outcome in patients with AHA. The data indicate that the presence of circulating anti-FVIII IgA antibodies at time of diagnosis is predictive of a poor recurrence-free survival, which was particular evident for patients with IgA antibody titers above the median of the study cohort. Moreover, AHA patients with circulating anti-FVIII IgA had an overall poor prognosis, achieving CR and OR less often and showing a decreased OS when compared with patients who were negative for anti-FVIII IgA. Interestingly, anti-FVIII IgA antibodies did not correlate with FVIII inhibitors and were only of low to medium apparent affinity. Most likely, these antibodies do not contribute to the neutralization of FVIII activity but rather represent distinct underlying immune mechanisms that maintain or even deteriorate the autoimmune pathology.
On the other hand, anti-FVIII IgG (1-4) antibodies at time of diagnosis showed a picture similar to what we described previously.10,11 Anti-FVIII IgG1 and IgG4 were the most prevalent IgG subclasses and presented with high antibody titers and high apparent affinities. All anti-FVIII IgG subclasses correlated in antibody titers with titers of FVIII inhibitors indicating that anti-FVIII IgG (1-4) were responsible for the neutralization of FVIII activity. However, none of the anti-FVIII IgG subclasses (1-4), analyzed at the time of diagnosis, were significant predictors of outcome. Patients with a secondary, low-affinity IgG1 antibody cluster tended to have a higher likelihood of PR and CR. The presence of a low-affinity IgG1 antibody cluster might be indicative of an earlier stage of the immune response when compared with patients with a single high-affinity IgG1 cluster. Moreover, there was a trend toward lower likelihood of PR and CR with high anti-FVIII antibody titers for most IgG subclasses, but these differences were either not statistically significant or disappeared after multivariate adjustment. We conclude that anti-FVIII IgG (1-4) and anti-FVIII IgA antibodies found at time of diagnosis in patients with AHA may have distinct biological functions.
The question arises whether circulating anti-FVIII IgA antibodies detected in AHA patients could be directly involved in the maintenance and deterioration of the autoimmune pathology or if they are solely surrogate markers of distinct underlying immune processes.
Arnason et al.14 recently reported an association of increased IgA levels with disease severity in patients suffering from immune thrombocytopenia. Patients with IgA levels above the median of the study cohort had a significantly increased chance of failing to respond to standard treatment (steroids, intravenous immunoglobulin, and intravenous anti-D) and were more likely to have a major bleed than patients with IgA levels lower than the median. Metzger et al.15 previously reported that high levels of IgA autoantibodies to tissue transglutaminase were associated with a highly increased mortality risk in patients with celiac disease. Ferrari et al.16 indicated a potential association of high-titer anti-ADAMTS13 IgA autoantibodies with increased mortality in patients with thrombotic microangiopathy. These previous reports together with our own data support the contention that IgA autoantibodies might contribute to the pathology of antibody-dependent autoimmune diseases.
Several authors demonstrated previously that circulating IgA antibodies have powerful anti-inflammatory properties but can also mediate proinflammatory activities.17-20 The molecular mechanisms for this rather paradoxical role of circulating IgA in immune regulation was first described by Pasquier et al in 2005.21 Monomeric binding of IgA to the Fc-α receptor I (FcαRI, CD89) expressed on myeloid cells (eg, monocytes, macrophages, dendritic cells, neutrophils, and eosinophils)22 results in the inhibition of proinflammatory activities. The FcαRI-mediated inhibition is mediated not by a common immunoreceptor tyrosine-based inhibition motif but by an associated immunoreceptor tyrosine-based activation (ITAM) motif. The FcαRI does not itself contain any immunoreceptor tyrosine-based inhibition motif or ITAM in its cytoplasmic tail but can be expressed in association with or without a Fc-γ receptor (FcγR) adaptor, which provides the ITAM motif.23 Binding of monomeric IgA to the FcαRI results in partial phosphorylation of its associated FcγR-ITAM, which creates an inhibitory ITAM motif (ITAMi). The ITAMi pathway involves recruitment of the tyrosine phosphatase SHP-1 and subsequent downstream inhibition of proinflammatory cell functions.24,25 In contrast, cross-linking of FcαRI by IgA bound to multimeric antigens or bound in immune complexes results in full phosphorylation of the associated FcγR-ITAM motif followed by the recruitment of the tyrosine kinase Syk, which in turn facilitates the activation of multiple targets, resulting in the downstream activation of proinflammatory effector functions.25,26 Examples for these proinflammatory effector functions are the induction of oxidative burst activities in neutrophils, an increase in antibody-dependent cytotoxic effector functions in macrophages, an increase in antigen presentation by antigen-presenting cells, a degranulation of neutrophils and eosinophils, or the release of proinflammatory cytokines by different myeloid cell populations.25,26 It appears that the FcαRI is a molecular switch that determines the proinflammatory or anti-inflammatory function of circulating IgA. One can imagine that immune complexes of IgA autoantibodies bound to distinct epitopes of human FVIII can result in cross-linking of FcαRI expressed on myeloid cells, thereby stimulating or amplifying innate immune activation, which could maintain or even deteriorate the autoimmune pathology in patients with AHA. On the other hand, FVIII bound to VWF can form a multimeric antigen, which could bind several molecules of IgA autoantibodies, thereby facilitating the cross-linking of FcαRI.
We conclude that IgA autoantibodies against FVIII could be directly involved in the maintenance and deterioration of the autoimmune pathology in patients with AHA. This would provide an explanation for the observation that patients with IgA autoantibodies against FVIII at time of diagnosis had a poor recurrence-free survival. Moreover, the ability of anti-FVIII IgA to maintain or even amplify innate immune activation could explain the overall poor prognosis of patients with anti-FVIII IgA. If confirmed in future clinical studies, our findings could help to better tailor immunosuppressive treatment in patients with AHA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted by the Thrombosis and Haemostasis Society of the German-speaking countries (GTH e.V.). Contributing investigators are listed in the supplemental Appendix. The authors acknowledge the contribution of all study sites, local study coordinators, and Susanne Bartels, central study coordinator at Hannover Medical School. Eva Budde is acknowledged for support in statistical analysis. The authors also thank Fatima Al-Awadi, Damir Fetahagic, Ellen Reift, and Virginia Martín Pontejo for excellent technical assistance in the immunologic sample analysis.
Authorship
Contribution: A.T., C.J.H., F.S., and B.M.R. designed the study, interpreted the data, and wrote the manuscript; C.J.H. was responsible for immunologic data management; A.T. and S.W. were responsible for clinical data management; A.T. and C.J.H. did the statistical analysis; P.K., S.G., K.H., J.H., J.G., R.E.S., and C.D. enrolled patients and collected and reviewed clinical data; and all authors critically reviewed the manuscript and approved of its publication.
Conflict-of-interest disclosure: Funding for this study was obtained from GTH e.V. and by unrestricted educational grants from Novo Nordisk Pharma GmbH (Mainz, Germany) and Baxter Deutschland GmbH (Baxalta) (Unterschleißheim, Germany). C.J.H., F.S., and B.M.R. are employees of Baxalta Innovations GmbH. The remaining authors declare no competing financial interests.
Correspondence: Andreas Tiede, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, Carl Neuberg Str 1, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal