In this issue of Blood, Denorme et al report on a potent thrombolytic activity of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) that restores the blood flow of acutely occluded middle cerebral artery (MCA) in a mouse model of stroke.1
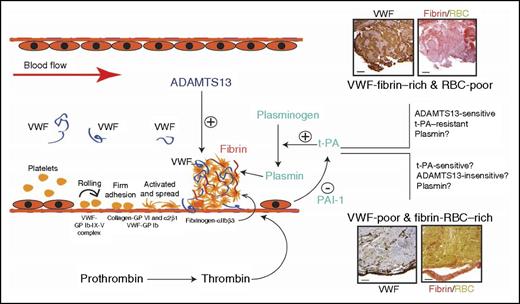
Potential roles of VWF in regulating the thrombolytic activity of t-PA. Results presented in this study are consistent with recent reports in linking VWF/ADAMTS13 to the pathogenesis of ischemic stroke. They suggest that VWF is involved in a subtype of ischemic stroke that has potentially more vascular injury. The VWF involvement may regulate t-PA efficacy and suggest alternative therapeutic targets. GP, glycoprotein; RBC, red blood cell. The histology images have been adapted from Figure 1 in the article by Denorme et al that begins on page 2337.
Potential roles of VWF in regulating the thrombolytic activity of t-PA. Results presented in this study are consistent with recent reports in linking VWF/ADAMTS13 to the pathogenesis of ischemic stroke. They suggest that VWF is involved in a subtype of ischemic stroke that has potentially more vascular injury. The VWF involvement may regulate t-PA efficacy and suggest alternative therapeutic targets. GP, glycoprotein; RBC, red blood cell. The histology images have been adapted from Figure 1 in the article by Denorme et al that begins on page 2337.
ADAMTS13 is a metalloprotease that cleaves the multimeric ligand von Willebrand factor (VWF) to reduce its adhesive activity. The genetic or acquired deficiency of this metalloprotease is the primary cause of thrombotic thrombocytopenic purpura (TTP), which is often presented with ischemic stroke. This study was inspired by the detection of substantial, but variable, amounts of VWF in thrombus tissues retrieved from patients with ischemic stroke or those with myocardial infarction.1-3 The authors show that in a mouse model, occlusive VWF-rich thrombi in MCA induced by localized injury were rapidly resolved by the infusion of the recombinant human ADAMTS13. The VWF-rich thrombi were resistant to thrombolysis by tissue plasminogen activator (t-PA), which catalyzes the conversion of plasminogen to plasmin to facilitate fibrinolysis. This study is important in 3 key aspects related to stroke pathogenesis and targeted therapies.
First, ischemic stroke has long been classified primarily as thrombotic and thromboembolic. The model used in the current study mimics acute thrombosis induced by localized vascular injury, as compared with thromboembolism that is common in stroke patients with severe atherosclerosis. This study raises a critical question as to whether ischemic stroke can be further categorized based on thrombus compositions, for example, as VWF-rich and VWF-poor. A more important question is whether such a classification can lead to better targeted and more effective stroke therapies. Since its regulatory approval nearly 20 years ago, t-PA has been widely used to treat acute ischemic stroke and, more recently, as a concurrent therapy for patients who undergo mechanical embolectomy. The effective t-PA treatment is indicative of fibrin-rich clots being the primary cause of ischemic stroke, but t-PA is less effective in patients with emergent large vessel occlusions from internal carotid artery and M1 middle MCA occlusion.4 The cause of this selective t-PA resistance remains poorly understood, but there has been a recent surge in research linking abnormal VWF/ADAMTS13 activities to the development of cerebral vascular occlusion in mouse models5-7 and in association studies of patients with ischemic stroke.8 A well-designed clinical trial will determine whether ADAMTS13 is effective for t-PA–resistant ischemic stroke, but a fundamental issue is in understanding and differentiating causes and compositions of cerebral thrombosis for targeted therapies. Recent studies indeed suggest that thrombus tissues dissected from patients with ischemic stroke vary structurally and in their VWF content.2
Second, the authors demonstrate that VWF-rich thrombi aspirated from sites of vascular occlusions are also extensively stained for fibrin.1,3 A recent study reports similar findings from tissues aspirated from sites of coronary artery occlusions and further identifies the VWF-fibrin–rich thrombi as resistant to thrombolytic treatment.3 The histological findings from thrombus tissues are consistent with the report that VWF multimers are integrated into polymerizing fibrin,9 indicating an extensive VWF-fibrin interaction in at least some occlusive thrombosis. These clinical and laboratory observations led to the question of whether fibrin bound to VWF becomes resistant to t-PA-plasmin, whereas VWF bound to fibrin remains sensitive to ADAMTS13. If this notion wins credence, VWF levels in thrombus tissue can serve as a molecular switch between t-PA and ADAMTS13 therapies, as schematically proposed (see figure).
Third, it is interesting to note that, although t-PA failed to resolve VWF-rich thrombi built at sites of vascular injury demonstrated in the current study,1 streptokinase and its catalytic product plasmin have been reported to disrupt VWF-platelet complexes formed on endothelial cells in a TTP-like setting.10 These apparent contradictory outcomes may indicate that injury to the vascular endothelial cells, which can be relatively minimal in thromboembolic stroke, is an important but less understood factor for stroke development and targeted therapeutics. One can speculate that the t-PA–mediated plasminogen activation is suppressed in vascular injury–mediated thrombosis, potentially by plasminogen activator inhibitor-1 (PAI-1) released from injured endothelial cells. In this regard, results from the current study also beg the question of whether plasmin, which also cleaves VWF,10 is effective in resolving vascular injury–induced thrombosis.
In summary, the study by Denorme et al suggests the need for further dissecting ischemic stroke to identify subtypes that are sensitive to t-PA or ADAMTS13 (and potentially anti-VWF agents). The findings also suggest therapeutic potentials of ADAMTS13 and anti-VWF agents in the treatment of acute coronary thrombosis, which more closely resembles the vascular occlusion modeled in the current study. Consistent with this possibility, a recent study reports that myocardial infarction patients, who are resistant to thrombolytic therapies, have higher fibrin and VWF contents in aspirated thrombus tissues, and elevated levels of PAIs and VWF in the circulation than those who are responsive to thrombolysis.3
Conflict-of-interest disclosure: The author declares no competing financial interests.