In this issue of Blood, Shoulars et al demonstrate the validity and reliability of a novel potency assay based on multiparameter flow cytometric enumeration of cells expressing aldehyde dehydrogenase (ALDH), CD45, and CD34 in tubing segments attached to cryopreserved cord blood units (CBUs) and its relevance to clinical practice.1
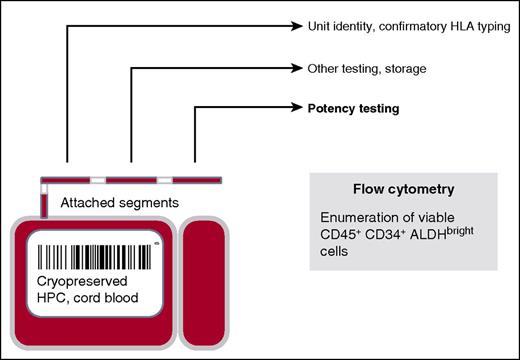
Cryopreserved CBUs have 2 detachable compartments for potential cell manipulations of the 20% or 80% fractions. Also, smaller aliquots of cryopreserved cells in detachable segments are available for quality control testing, such as confirmatory HLA typing and analysis of the unit’s potency. HPC, hematopoietic progenitor cell.
Cryopreserved CBUs have 2 detachable compartments for potential cell manipulations of the 20% or 80% fractions. Also, smaller aliquots of cryopreserved cells in detachable segments are available for quality control testing, such as confirmatory HLA typing and analysis of the unit’s potency. HPC, hematopoietic progenitor cell.
Human umbilical cord blood (UCB) is an established alternative source of hematopoietic stem and progenitor cells that are capable of reconstituting hematopoiesis after intensive myeloablative therapy. UCB is immediately available, poses no risk to the donor, is less restricted in HLA-matching requirements, and is associated with a potent graft-versus-leukemia effect. As of January 2016, an estimated 686 197 CBUs have been stored, and nearly 40 000 transplants using UCB have been performed worldwide.2,3
Unfortunately, CBUs do not uniformly engraft.4 Reports in children and adults suggest that UCB transplantation is typically associated with a marked delay in hematopoietic recovery, with about 10% to 20% of patients never engrafting at all. In addition, speed and incidence of hematopoietic recovery are associated with the dose of nucleated cells, CD34+ cells, and viable CD34+ cells; however, the granulocyte-macrophage colony-forming unit (CFU-GM) dose from the thawed product may be the best predictor.5-8 Although CFU-GM provides an in vitro “functional” assessment, it has been difficult to standardize across centers and even between individuals within a given laboratory. In addition, evaluation of CFU-GM requires 10 to 14 days and is most often examined on the CBU after it has been thawed on the day of transplant, thereby limiting its usefulness. For UCB, the ideal potency bioassay would be fast, reproducible, and reflective of the thawed UCB product to be used for transplantation, but with results known prior to unit selection. This could be accomplished by testing an attached segment (see figure).
Because the transplant team is often faced with multiple CBUs suitable for the patient on the basis of HLA match and minimum cell dose threshold, such as 2.5 × 107 nucleated cells per kilogram recipient body weight, the recent study by Shoulars et al provides us with a new tool for identifying the best of the available CBUs. Based on the known functionality of ALDH expression in primitive CD34 hematopoietic cells, enriching for cells with repopulation potential,9 the investigators’ studies provide the first evidence supporting the reproducibility and validity of this flow-based method, which highly correlates with the thawed product’s CFU content.
Clearly, these results support further evaluation by cord blood banks and transplant centers. If confirmed, it is conceivable that such a potency assay, tailored to the assessment of cryopreserved CBUs, could be used to assess product quality between cord blood banks and manufacturing consistency within a bank, especially as approaches for cell processing, progenitor enrichment, and cryopreservation evolve. For the transplant center, however, being able to choose the CBU with the greatest engraftment potential is paramount. If the total number or dose of ALDHbright and viable CD45+/CD34+ cells do indeed correlate with the speed of recovery and engraftment after UCB transplantation, this may be an important means for reducing the risks of delayed hematopoietic recovery and graft failure, which in turn could enhance survival and reduce hospital costs. Furthermore, this could easily be incorporated into studies of ex vivo expansion culture either as an approach to identify which patients are most benefited by the infusion of expanded cells or as a way to optimize which units might have the greatest expansion potential.
Although the report is indeed promising, it is early. As cited by the investigators, there are technical hurdles. Refinements in operating procedures may be needed to ensure the validity of the results from the attached segment. For example, studies by the France Greffe de Moelle registry bring this into question.10 An analysis of 117 CBUs, revealed >10% differences in the total nucleated cell viability between the thawed distal segment and the thawed bag in 64 cases. Although it was more likely that viability was lower in the segment than in the bag, such a result could inappropriately exclude good CBUs. Discordance between the thawed segment and CBU could be related to differences in the way the CBU and the segment itself are prepared and thawed.
In conclusion, the study by Shoulars et al is of substantial interest with far reaching implications in the cord blood field. For the cord blood banks, the next steps are to demonstrate that such a method is transferable and can be validated in a compliant environment. For the transplant centers, the next step is to determine the predictive value of viable CD45+/CD34+ ALDHbright cell number on hematopoietic recovery after transplantation.
Conflict-of-interest disclosure: The author declares no competing financial interests.