Key Points
Antibodies produced by donor B cells are required for thymic and lymphoid damage in mice with chronic GVHD.
Antibody-producing donor B cells associate with infiltration of Th17 cells in the skin and perpetuation of cutaneous chronic GVHD in mice.
Abstract
Cutaneous sclerosis is one of the most common clinical manifestations of chronic graft-versus-host disease (cGVHD). Donor CD4+ T and B cells play important roles in cGVHD pathogenesis, but the role of antibodies from donor B cells remains unclear. In the current studies, we generated immunoglobulin (Ig)Hµγ1 DBA/2 mice whose B cells have normal antigen-presentation and regulatory functions but cannot secrete antibodies. With a murine cGVHD model using DBA/2 donors and BALB/c recipients, we have shown that wild-type (WT) grafts induce persistent cGVHD with damage in the thymus, peripheral lymphoid organs, and skin, as well as cutaneous T helper 17 cell (Th17) infiltration. In contrast, IgHµγ1 grafts induced only transient cGVHD with little damage in the thymus or peripheral lymph organs or with little cutaneous Th17 infiltration. Injections of IgG-containing sera from cGVHD recipients given WT grafts but not IgG-deficient sera from recipients given IgHµγ1 grafts led to deposition of IgG in the thymus and skin, with resulting damage in the thymus and peripheral lymph organs, cutaneous Th17 infiltration, and perpetuation of cGVHD in recipients given IgHµγ1 grafts. These results indicate that donor B-cell antibodies augment cutaneous cGVHD in part by damaging the thymus and increasing tissue infiltration of pathogenic Th17 cells.
Introduction
Chronic graft-versus-host disease (cGVHD) is an autoimmune syndrome after allogeneic hematopoietic cell transplantation (HCT).1-5 The clinical symptoms of cGVHD are highly variable, but sclerosis of the skin and fascia is one of the most debilitating manifestations.6,7 Donor CD4+ T and B cells play important roles in cGVHD pathogenesis.8,9 Donor B cells in cGVHD patients are aberrantly activated, and their role in cGVHD pathogenesis is proposed to involve abnormalities in their antigen-presenting cell function, antibody production, and regulatory function.10,11 Reduction of interleukin-10 (IL-10)–producing regulatory B cells was found in cGVHD patients and murine models.12-14 We reported that donor B cells augmented clonal expansion of pathogenic CD4+ T cells via their antigen-presenting cell function and augmented sclerotic cGVHD of the skin.15 Immunoglobulin G (IgG) deposition in the skin has been observed in murine models and in humans with cGVHD.9,16,17 Srinivasan et al showed that donor B-cell–derived antibodies augmented development of bronchiolitis obliterans in a murine model of cGVHD characterized by pulmonary fibrosis without cutaneous sclerosis.18 In this model, recipient germinal centers (GCs) were enlarged, and blockade of GC formation prevented induction of cGVHD.19 On the other hand, cGVHD patients often have lymphopenia and cutaneous sclerosis.2,20 Thus, the role of IgG antibodies from donor B cells in the pathogenesis of cutaneous cGVHD in recipients with lymphopenia remains unclear.
Although previous studies suggested that induction of cGVHD in murine models required specific strain combinations,21 our recent studies have shown that the key for induction of cGVHD is not the particular strain combination, but the number of donor T cells in the graft. With appropriate numbers of donor T cells in the graft, recipients can survive for >40 to 60 days, allowing manifestations of cGVHD to emerge.16 Murine cGVHD recipients develop a systemic autoimmune syndrome with features characteristic of cGVHD in humans, including autoantibodies, cutaneous sclerosis, damage in the salivary lacrimal glands, and lymphocytic bronchiolitis.2,15,16 Consistently, we have observed similar cGVHD cutaneous sclerosis and damage in salivary and lacrimal glands in BALB/c recipients given major histocompatibility complex (MHC)-mismatched C57BL/6 or MHC-matched DBA/2 transplants 40 to 60 days after HCT,15,16 and donor B cells play an important role in cGVHD pathogenesis in both models.22
In the current studies, we used IgHµγ1 DBA/2 donor mice whose B cells do not secrete antibodies but otherwise have normal antigen-presentation and regulatory functions. We found that donor B-cell–derived antibodies damage the thymus and lymphoid tissue, augment T helper 17 cell (Th17) infiltration in the skin, and perpetuate sclerotic cGVHD of the skin.
Methods
DBA/2 and BALB/c mice were purchased from the National Cancer Institute Animal Production Program (Frederick, MD). IgHμγ1 DBA/2 mice were generated by backcrossing IgHμγ1 BALB/c mice to DBA/2 for 10 generations. IgHμγ1 BALB/c mice23 were provided by Dr Klaus Rajewski at Harvard University. Mice were maintained in a pathogen-free room at City of Hope Animal Research Center. All experiments were approved by the City of Hope institutional animal care and use committee. Induction and assessment of graft-versus-host disease (GVHD), antibodies, flow cytometry analysis and sorting, histopathology and histoimmunofluoresent staining, real-time polymerase chain reaction, and statistical analysis are described in previous publications15,16,24 and in supplemental Methods (available on the Blood Web site).
Results
Antibody-producing donor B cells are required for persistence of cGVHD tissue damage, but are not required to initiate tissue damage
By backcrossing IgHµγ1 BALB/c mice23 to DBA/2 mice, we established IgHµγ1 DBA/2 mice whose B cells do not secrete antibodies but otherwise have normal antigen-presentation and regulatory functions. As shown in supplemental Figure 1, the IgHµγ1 DBA/2 mice had only IgMhiIgD− B cells with no IgMloIgDhi B cells and had little serum IgM or IgG (supplemental Figure 1A-B). The mice had similar percentages of transitional 1, transitional 2/marginal zone, and follicular B cells as compared with wild-type (WT) litermate controls (supplemental Figure 1C). Before and after HCT, IgHµγ1 B cells expressed similar levels of CD40 and MHCII, and they had similar expression of IL-10 messenger RNA (mRNA) as compared with control WT B cells (supplemental Figure 1D-E). Furthermore, CD25+ cell-depleted spleen (CD25−-SPL) cells from IgHµγ1 donors showed no suppression effect on cGVHD, as addition of WT or IgHµγ1 donor CD25−-SPL cells both augmented induction of cGVHD with no significant difference in cutaneous cGVHD scores (supplemental Figure 1F). These results indicate that IgHµγ1 B cells have normal antigen-presentation and regulatory functions.
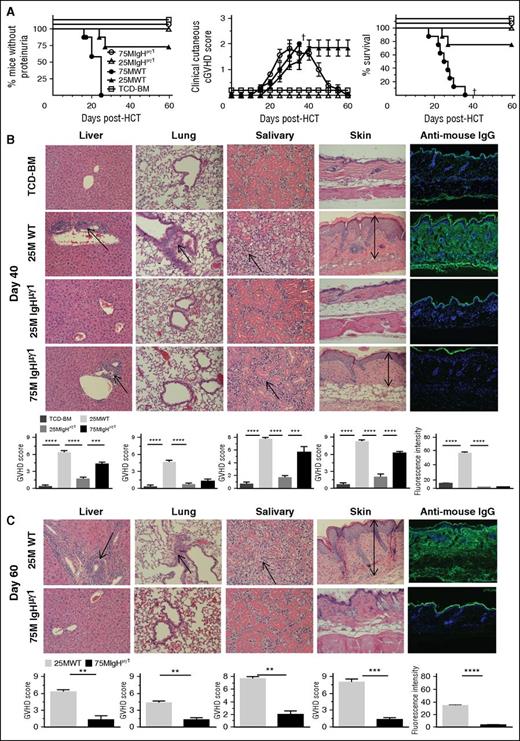
Consistent with our previous publications,9,15,22 we used CD25−-SPL cells to induce GVHD, and depletion of CD25+ cells did not significantly change the composition of CD4+ T and B cells in the grafts (supplemental Figure 2). Accordingly, T-cell–depleted bone marrow (TCD-BM) (2.5 × 106) alone or with addition of CD25−-SPL (25 or 75 × 106) from WT or IgHµγ1 DBA/2 mice were transplanted into lethally irradiated (850 cGy total body irradiation [TBI]) BALB/c recipients. Recipients given TCD-BM from WT or IgHµγ1 DBA/2 donors showed no signs of GVHD and were combined together as the TCD-BM control group. Recipients given 75 × 106 WT CD25−-SPL cells all developed proteinuria by ∼25 days after HCT, and all died with proteinuria and cutaneous GVHD by 35 days after HCT (Figure 1A). In contrast, recipients given 75 × 106 IgHµγ1 CD25−-SPL cells did not develop proteinuria, and all survived beyond day 60 (P < .01, Figure 1A). These recipients showed progressive cutaneous GVHD with hair loss at ∼25 days after HCT, with clinical cutaneous cGVHD scores reaching a plateau by ∼35 days after HCT. Cutaneous GVHD gradually subsided, hair regrew, and clinical manifestations resolved by day 60 (Figure 1A). Approximately 25% of the recipients given 25 × 106 WT CD25−-SPL cells developed proteinuria and died by ∼30 days after HCT. The remaining recipients survived for >60 days with persistent cutaneous GVHD (Figure 1A). In contrast, the recipients given 25 × 106 IgHµγ1 CD25−-SPL cells showed no signs of proteinuria or cutaneous GVHD, and all survived beyond 60 days (Figure 1A).
Antibody-producing donor B cells are not required to initiate tissue damage, but they are required for persistence of tissue damage. BALB/c recipients were lethally irradiated (850 cGy TBI) and given high-dose (75 × 106) and low-dose (25 × 106) CD25-depleted splenocytes and TCD-BM (2.5 x106) cells from WT or IgHμγ1 DBA/2 donors. Recipients given TCD-BM (2.5 × 106) from WT or IgHμγ1 DBA/2 donors alone were combined and used as negative controls for GVHD. Recipients were monitored for GVHD development, including proteinuria, clinical cutaneous GVHD, and survival († indicates death of all recipients in a group). (A) Percentage of mice without proteinuria, clinical cutaneous GVHD score, and percentage of survival; N = 8 from 2 replicate experiments. (B) On day 40 after HCT, histopathology of the GVHD target tissues liver, lung, salivary gland, and skin, and antibody deposition in the skin was evaluated. A representative photomicrograph (original magnification ×200 for histopathology and ×100 for IgG deposition); means ± standard error (SE) (N = 6) of histopathology scores are shown. Arrows indicate the following: infiltration in the liver, lymphocytic bronchiolitis, infiltration and loss of ductal structure in the salivary gland, and hyperplasia in the epidermis, expansion of dermis, and loss of subcutaneous fat. Antibody deposition in the skin was quantified by the intensity of anti-mouse IgG–fluorescein isothiocyanate (FITC) in 5 different levels of 4 examined mice at each group. (C) On day 60 after HCT, histopathology of the GVHD target tissues liver, lung, salivary gland, skin, and antibody deposition in the skin was evaluated. A representative photomicrograph (original magnification, ×200 for histopathology and ×100 for IgG deposition); means ± SE (N = 6) of histopathology scores or means ± SE (N = 4) of anti-IgG–FITC intensity are shown. Arrows are described in panel B (**P < .01, ***P < .001, ****P < .0001).
Antibody-producing donor B cells are not required to initiate tissue damage, but they are required for persistence of tissue damage. BALB/c recipients were lethally irradiated (850 cGy TBI) and given high-dose (75 × 106) and low-dose (25 × 106) CD25-depleted splenocytes and TCD-BM (2.5 x106) cells from WT or IgHμγ1 DBA/2 donors. Recipients given TCD-BM (2.5 × 106) from WT or IgHμγ1 DBA/2 donors alone were combined and used as negative controls for GVHD. Recipients were monitored for GVHD development, including proteinuria, clinical cutaneous GVHD, and survival († indicates death of all recipients in a group). (A) Percentage of mice without proteinuria, clinical cutaneous GVHD score, and percentage of survival; N = 8 from 2 replicate experiments. (B) On day 40 after HCT, histopathology of the GVHD target tissues liver, lung, salivary gland, and skin, and antibody deposition in the skin was evaluated. A representative photomicrograph (original magnification ×200 for histopathology and ×100 for IgG deposition); means ± standard error (SE) (N = 6) of histopathology scores are shown. Arrows indicate the following: infiltration in the liver, lymphocytic bronchiolitis, infiltration and loss of ductal structure in the salivary gland, and hyperplasia in the epidermis, expansion of dermis, and loss of subcutaneous fat. Antibody deposition in the skin was quantified by the intensity of anti-mouse IgG–fluorescein isothiocyanate (FITC) in 5 different levels of 4 examined mice at each group. (C) On day 60 after HCT, histopathology of the GVHD target tissues liver, lung, salivary gland, skin, and antibody deposition in the skin was evaluated. A representative photomicrograph (original magnification, ×200 for histopathology and ×100 for IgG deposition); means ± SE (N = 6) of histopathology scores or means ± SE (N = 4) of anti-IgG–FITC intensity are shown. Arrows are described in panel B (**P < .01, ***P < .001, ****P < .0001).
Compared with TCD-BM control, recipients given 25 × 106 WT CD25−-SPL cells showed typical histopathology of cGVHD at day 40,15,16 including (1) lymphocytic infiltration in hepatic portal triads; (2) lymphocytic bronchiolitis; (3) ductal infiltration and loss of serous secretion in the salivary gland; and (4) epidermal hyperplasia, expansion of dermis, and loss of subcutaneous fat, and IgG antibody deposition in the skin (Figure 1B). This pathology persisted to 60 days after HCT (Figure 1C), and cutaneous involvement was characterized by significant collagen deposition as compared with TCD-BM recipients (P < .01, supplemental Figure 3). Recipients given 25 × 106 IgHµγ1 CD25−-SPL cells showed little tissue damage (P < .01, Figure 1B). Grafts containing 75 × 106 IgHµγ1 CD25−-SPL cells induced tissue pathology in the liver, salivary gland, and skin, but not the lung at 40 days after HCT (Figure 1B), but the damage resolved by day 60 after HCT (Figure 1C). IgG deposition was present in the skin of recipients given WT-SPL but not IgHµγ1-SPL cells at 40 and 60 days after HCT (P < .01, Figure 1B-C). These results suggest that IgG from donor B cells is not required to initiate cGVHD tissue damage in the liver, salivary glands, or skin but is required for persistence of tissue damage.
Antibody-producing donor B cells augment lymphoid tissue damage and loss of GCs in cGVHD recipients
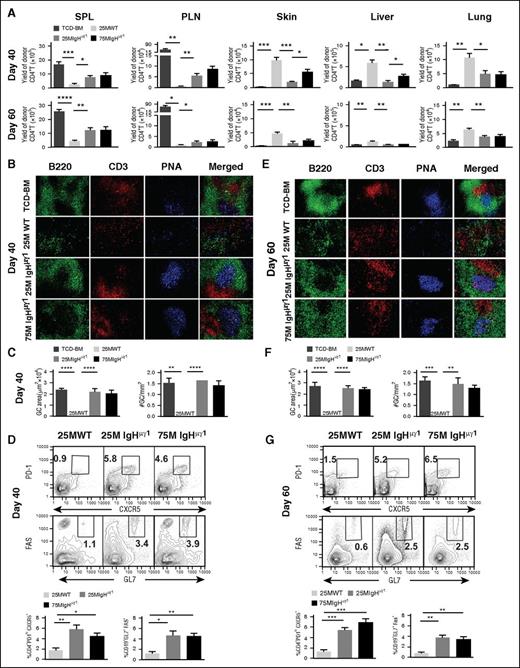
As mentioned in the “Introduction,” other investigators have reported that cGVHD development was associated with enlarged GCs in B-cell follicles, using a murine model of lung cGVHD.19,25 We tested whether these abnormalities are present in our murine model. First, we compared CD4+ T-cell yield in the spleen, peripheral lymph nodes (PLNs), skin, liver, and lung of different cGVHD recipients at 40 and 60 days after HCT. For brevity, recipients given TCD-BM alone without clinical signs of cGVHD are described as “TCD-BM-no-cGVHD recipients”; recipients given 25 × 106 WT CD25−-SPL cells with persistent clinical signs of cGVHD are described as “WT-cGVHD recipients”; recipients given 25 × 106 IgHµγ1 CD25−-SPL cells with little clinical signs of cGVHD are described as as “IgHµγ1-no-cGVHD recipients”; recipients given 75 × 106 IgHµγ1 CD25−-SPL cells with transient cGVHD are described as “IgHµγ1-transient-cGVHD recipients”. As compared with control TCD-BM-no-cGVHD recipients, WT-cGVHD recipients had a marked reduction of donor T cells in the spleen and PLNs but a marked increase of infiltrating T cells in the skin, liver, and lung at day 40 (P < .01-.05, Figure 2A top row). As compared with WT-cGVHD recipients, IgHµγ1-no-cGVHD recipients had a marked increase of T-cell numbers in the spleen and PLNs, and significantly lower numbers of T cells in the skin, liver, and lung (P < .01-.05, Figure 2A top row). Similar trends were observed at day 60 (Figure 2A bottom row).
Antibody-producing donor B cells augment lymphoid tissue damage and loss of GCs in cGVHD recipients. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Kinetic changes in donor CD4+ T-cell expansion in the spleen and lymph nodes and infiltration in the GVHD target tissues (skin, liver, and lung) at 40 and 60 days after HCT. Spleen, PLNs, skin, liver and lung of recipients were harvested for analysis of donor CD4+ T yield. Means ± SE of the yield of CD5.1+TCRβ+CD4+ T cells are shown. N = 4 from at least 2 replicate experiments. (B,E) Frozen spleen sections from TCD-BM-no-cGVHD, WT-cGVHD, IgHμγ1-no-cGVHD, and IgHμγ1-transient-cGVHD recipients at days 40 and 60 after HCT were analyzed by immunofluorescent staining of GC structures. Spleen sections were stained for B220 (green), CD3 (red), peanut agglutinin (PNA; blue), 40 (B) and 60 (E) days after HCT. The blue area in the merged picture is the GC. A photomicrograph (original magnification, ×200) representative of 4 replicate experiments is shown. (C,F) The size of the GC at days 40 and 60 after HCT was quantified by measuring the area of PNA staining in Photoshop C3, and the frequencies of GCs at days 40 and 60 after HCT were quantified by counting the number of GCs per mm2 of spleen section. Means ± SE of the size and frequency of GCs are shown. N = 4 from at least 2 replicate experiments. (D,G) Frequency of TFH cells (defined as PD-1hiCXCR5+CD4+ T cells) and GC B cells (defined as GL7+Fas+ B cells). On days 40 (D) and 60 (G) after HCT, splenocytes were stained with anti-CD4, T-cell receptor β (TCRβ), programmed cell death 1 (PD-1), and CXC chemokine receptor 5 (CXCR5) monoclonal antibodies (mAbs), and gated CD4+TCRβ+ cells are shown as PD-1 vs CXCR5. Splenocytes were also stained with anti-CD19, GL7, and Fas mAbs, and gated CD19+ cells are shown as Fas vs GL7. CXCR5+PD-1+ TFH and Fas+GL7+ GC B cells are gated. Representative staining profiles are shown. Means ± SE of the frequency of TFH cells and GC B cells are shown. N = 4 from at least 2 replicate experiments (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Antibody-producing donor B cells augment lymphoid tissue damage and loss of GCs in cGVHD recipients. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Kinetic changes in donor CD4+ T-cell expansion in the spleen and lymph nodes and infiltration in the GVHD target tissues (skin, liver, and lung) at 40 and 60 days after HCT. Spleen, PLNs, skin, liver and lung of recipients were harvested for analysis of donor CD4+ T yield. Means ± SE of the yield of CD5.1+TCRβ+CD4+ T cells are shown. N = 4 from at least 2 replicate experiments. (B,E) Frozen spleen sections from TCD-BM-no-cGVHD, WT-cGVHD, IgHμγ1-no-cGVHD, and IgHμγ1-transient-cGVHD recipients at days 40 and 60 after HCT were analyzed by immunofluorescent staining of GC structures. Spleen sections were stained for B220 (green), CD3 (red), peanut agglutinin (PNA; blue), 40 (B) and 60 (E) days after HCT. The blue area in the merged picture is the GC. A photomicrograph (original magnification, ×200) representative of 4 replicate experiments is shown. (C,F) The size of the GC at days 40 and 60 after HCT was quantified by measuring the area of PNA staining in Photoshop C3, and the frequencies of GCs at days 40 and 60 after HCT were quantified by counting the number of GCs per mm2 of spleen section. Means ± SE of the size and frequency of GCs are shown. N = 4 from at least 2 replicate experiments. (D,G) Frequency of TFH cells (defined as PD-1hiCXCR5+CD4+ T cells) and GC B cells (defined as GL7+Fas+ B cells). On days 40 (D) and 60 (G) after HCT, splenocytes were stained with anti-CD4, T-cell receptor β (TCRβ), programmed cell death 1 (PD-1), and CXC chemokine receptor 5 (CXCR5) monoclonal antibodies (mAbs), and gated CD4+TCRβ+ cells are shown as PD-1 vs CXCR5. Splenocytes were also stained with anti-CD19, GL7, and Fas mAbs, and gated CD19+ cells are shown as Fas vs GL7. CXCR5+PD-1+ TFH and Fas+GL7+ GC B cells are gated. Representative staining profiles are shown. Means ± SE of the frequency of TFH cells and GC B cells are shown. N = 4 from at least 2 replicate experiments (*P < .05, **P < .01, ***P < .001, ****P < .0001).
IgHµγ1-no-cGVHD and IgHµγ1-transient-cGVHD recipients had similar numbers of T cells in the spleen, PLNs, and lung at days 40 and 60. However, the numbers of T cells in the skin and liver were higher in IgHµγ1-transient-cGVHD recipients than in IgHµγ1-no-cGVHD recipients at day 40 (P< .05, Figure 2A top row), but the infiltration in the former disappeared by day 60 (Figure 2A bottom row). Taken together, these results indicate that in the absence of antibodies from donor B cells, high numbers of donor T cells induce only transient cGVHD and suggest that antibodies from donor B cells directly or indirectly augment induction of cGVHD with a marked decrease of CD4+ T cells in lymphoid tissues but a marked increase in GVHD target tissues.
We analyzed splenic lymphoid structure and GCs by histoimmunofluorescent staining and measured the percentage of follicular helper T (TFH) and GC B cells by flow cytometry. At day 40, TCD-BM-no-cGVHD recipients had B220+ B-cell follicular structures with a CD3+ T-cell zone and PNA+ GC, and the GC was located in the center of B-cell follicle (Figure 2B first row), but WT-cGVHD recipients showed destruction of B-cell follicles and lack of GCs (Figure 2B second row). Testing was done at days 15 and 30 in order to determine whether lymphoid follicles and GCs were destroyed or were not formed in cGVHD recipients. TCD-BM-no-cGVHD recipients had GCs at day 15 and day 30 after HCT. WT-cGVHD recipients also formed lymphoid follicules and GCs at day 15, and the GCs appeared to be larger than in TCD-BM-no-cGVHD recipients, but they disappeared by day 30 after HCT (supplemental Figure 4). These results indicate that injected donor CD4+ T and B cells can form lymphoid follicles and GCs by day 15, but they are destroyed by day 30 after HCT, at the onset of cGVHD.
In contrast to WT-cGVHD recipients, IgHµγ1-no-cGVHD and IgHµγ1-transient-cGVHD recipients both showed B-cell follicles and GCs at 40 days after HCT (Figure 2B third and fourth rows), and their GC area and size was similar to that of TCD-BM-no-cGVHD recipients, which was markedly greater than that of WT-cGVHD recipients (P < .001), as the GC area and size in the latter recipients was not detectable (Figure 2C). We further analyzed the percentage of CXCR5+PD-1highCD4+ TFH cells and Fas+GL7+CD19+ GC B cells. At day 40, WT-cGVHD recipients had very few (∼1%) TFH or GC B cells (Figure 2D). IgHµγ1-no-cGVHD recipients or IgHµγ1-transient-cGVHD recipinets had similar percentages of TFH (∼6%) and GC B cells (∼4%), and both were markedly higher than in WT-cGVHD recipients (P < .05, Figure 2D). Similar lymphoid structures and percentages of TFH and GC B cells were observed at day 60 (Figure 2E-G). Taken collectively, these results suggest that IgG antibodies from donor B cells directly or indirectly contribute to destruction of lymphoid follicles and GCs in WT-cGVHD recipients with lymphopenia.
Antibody-producing donor B cells associate with thymus damage in cGVHD recipients
One cause of lymphopenia in cGVHD is lack of thymic regeneration,20 and the percentage and yield of CD4+CD8+ thymocytes is the best indication of thymic regeneration.26 As compared with TCD-BM-no-cGVHD recipients, WT-cGVHD recipients had marked reduction in percentage and yield of CD4+CD8+ thymocytes at days 40 and 60 (P < .01, Figure 3A). In contrast, the percentages and yields of CD4+CD8+ thymocytes in IgHµγ1-no-cGVHD recipients and IgHµγ1-transient-cGVHD recipients were similar, not significantly different from TCD-BM-no-cGVHD recipients (Figure 3A). WT CD25−-SPL cells caused marked loss of medullary thymic epithelial cells (mTECs) as compared with TCD-BM controls as judged by flow cytometry and histoimmunofluoresent staining (P< .01, Figure 3B-C). IgHµγ1 CD25−-SPL cells caused little mTEC damage at the same time points (Figure 3B-C). Thymic damage in the recipients given WT CD25−-SPL cells was associated with IgG antibody deposition in the thymus (Figure 3D). These results suggest that IgG antibodies from donor B cells directly or indirectly contribute to thymic mTEC damage in cGVHD recipients.
Antibody-producing donor B cells associate with thymus damage in cGVHD recipients. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Analysis of CD4+CD8+ double-positive thymocytes at 40 and 60 days after HCT. Staining profiles representative of 4 replicate experiments are shown, Means ± SE are displayed for percentage and yield of double-positive thymocytes at 40 and 60 days after HCT. (B) On days 40 and 60 after HCT, BALB/C recipient thymus cell suspensions were measured for the percentage of mTECs. Gated thymic epithelial cells (TECs) are shown as UEA I (medullary TEC [mTEC] marker) vs Ly51 (cortical TEC marker). A flow cytometry pattern representative of 4 replicate experiments is shown. Means ± SE (N = 4) of the percentage of mTECs are shown. (C) On day 40 after HCT, thymus sections were analyzed by histoimmunofluorescent staining for cytokeratin 8 (red, cortical epithelial cells) and UEA-I (green, mTEC). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown. (D) On day 40 after HCT, thymus sections were evaluated by histoimmunofluorescent staining with anti-mouse IgG-FITC (green) and 4,6 diamidino-2-phenylindole (DAPI) (blue, nucleus). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown (**P < .01, ***P < .001, ****P < .0001).
Antibody-producing donor B cells associate with thymus damage in cGVHD recipients. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Analysis of CD4+CD8+ double-positive thymocytes at 40 and 60 days after HCT. Staining profiles representative of 4 replicate experiments are shown, Means ± SE are displayed for percentage and yield of double-positive thymocytes at 40 and 60 days after HCT. (B) On days 40 and 60 after HCT, BALB/C recipient thymus cell suspensions were measured for the percentage of mTECs. Gated thymic epithelial cells (TECs) are shown as UEA I (medullary TEC [mTEC] marker) vs Ly51 (cortical TEC marker). A flow cytometry pattern representative of 4 replicate experiments is shown. Means ± SE (N = 4) of the percentage of mTECs are shown. (C) On day 40 after HCT, thymus sections were analyzed by histoimmunofluorescent staining for cytokeratin 8 (red, cortical epithelial cells) and UEA-I (green, mTEC). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown. (D) On day 40 after HCT, thymus sections were evaluated by histoimmunofluorescent staining with anti-mouse IgG-FITC (green) and 4,6 diamidino-2-phenylindole (DAPI) (blue, nucleus). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown (**P < .01, ***P < .001, ****P < .0001).
Antibody-producing donor B cells associate with augmented Th17 infiltration and expansion in the skin
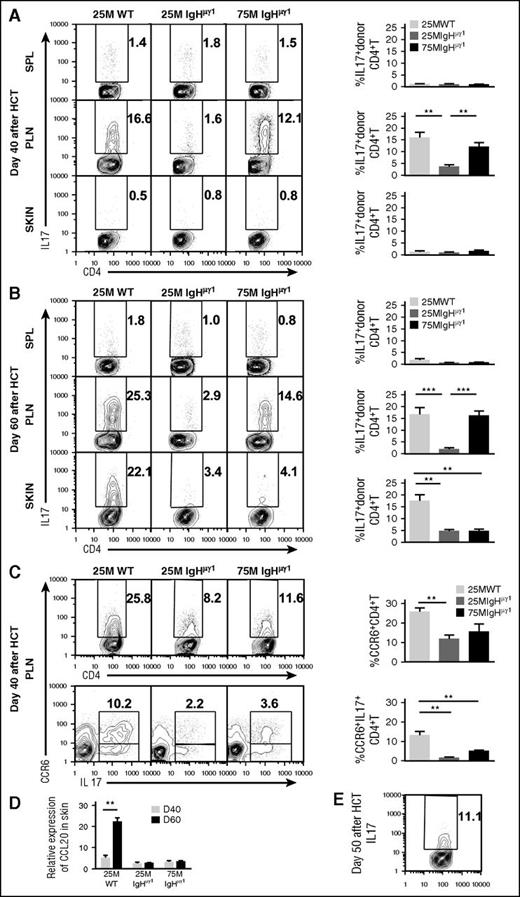
Both interferon-γ (IFN-γ)–producing Th1 and IL-17–producing Th17 cells are involved in the pathogenesis of cutaneous GVHD in animal models and humans.27-35 Our previous report showed that B cells augmented Th17 expansion in the lung but not in the skin at 40 days after HCT.15 Whether donor B cells and their antibodies affect Th17 expansion in the skin beyond 40 days after HCT has not been investigated. Thus, we compared the percentage of Th1 and Th17 in the spleen, PLNs, and skin of WT-cGVHD, IgHµγ1-no-cGVHD, and IgHµγ1-transient-cGVHD recipients. At 40 days after HCT, the early peak of cutaneous GVHD development, the spleens of all recipients contained 10% to 15% Th1 and 1% to 2% Th17 cells (supplemental Figure 5A; Figure 4A). The PLNs of both WT-cGVHD and IgHµγ1-transient-cGVHD recipients contained ∼15% Th1 and Th17 cells, but the PLNs of IgHµγ1-no-cGVHD recipients had only ∼5% Th1 and ∼2% Th17, which was significantly lower than in the other 2 groups (P < .05; supplemental Figure 5A; Figure 4A). The skin of WT-cGVHD and IgHµγ1-no-cGVHD recipients had similar percentages of Th1 (∼10%), but the skin of IgHµγ1-transient-cGVHD recipients had ∼30% Th1, which was approximately threefold higher than in the other 2 groups (P < .05, supplemental Figure 5A). In all 3 groups, the skin contained only ∼1% of Th17 cells (Figure 4A). These results suggest that at day 40, Th1 cells play an important role in skin GVHD pathogenesis, and that antibodies from donor B cells can directly or indirectly augment Th1-mediated pathogenesis.
Antibody-producing donor B cells associate with augmented Th17 infiltration and increased expression of CCL20 in the skin. HCT was carried out with BALB/c recipients as described in Figure 1. (A-B) Percentage of IL-17+ cells among donor CD4+ T cells at days 40 (A) and 60 (B) after HCT. Donor CD5.1+CD4+ T cells from spleen, PLNs, and skin were analyzed with intracellular staining of IL-17. Cells were gated on CD5.1+TCRβ+ and displayed as IL-17 vs CD4. Representative patterns and means ± SE of the percentage of donor IL17+CD4+ T cells in the spleen, PLNs, and skin are shown. N = 4-6 from at least 2 replicate experiments. (C) Percentage of CCR6+ donor CD4+T cells and CCR6+IL17+ double-positive donor CD4+ T cells at day 40 after HCT. Representative patterns and means ± SE of the percentage of donor CCR6+CD4+ T cells or CCR6+IL17+ double-positive CD4+ T cells in PLNs are shown. N = 4 from at least 2 replicate experiments for each group. (D) Means ± SE of CCL20 chemokine expression in skin at 40 and 60 days after HCT. Relative gene expression levels were normalized within each sample to the housekeeping gene GAPDH. N = 3 from 3 replicate experiments with triplicates (**P < .01, ***P < .001). (E) Representative staining pattern of donor IL-17+CD4+ T cells in skin at day 50 after HCT. N = 3.
Antibody-producing donor B cells associate with augmented Th17 infiltration and increased expression of CCL20 in the skin. HCT was carried out with BALB/c recipients as described in Figure 1. (A-B) Percentage of IL-17+ cells among donor CD4+ T cells at days 40 (A) and 60 (B) after HCT. Donor CD5.1+CD4+ T cells from spleen, PLNs, and skin were analyzed with intracellular staining of IL-17. Cells were gated on CD5.1+TCRβ+ and displayed as IL-17 vs CD4. Representative patterns and means ± SE of the percentage of donor IL17+CD4+ T cells in the spleen, PLNs, and skin are shown. N = 4-6 from at least 2 replicate experiments. (C) Percentage of CCR6+ donor CD4+T cells and CCR6+IL17+ double-positive donor CD4+ T cells at day 40 after HCT. Representative patterns and means ± SE of the percentage of donor CCR6+CD4+ T cells or CCR6+IL17+ double-positive CD4+ T cells in PLNs are shown. N = 4 from at least 2 replicate experiments for each group. (D) Means ± SE of CCL20 chemokine expression in skin at 40 and 60 days after HCT. Relative gene expression levels were normalized within each sample to the housekeeping gene GAPDH. N = 3 from 3 replicate experiments with triplicates (**P < .01, ***P < .001). (E) Representative staining pattern of donor IL-17+CD4+ T cells in skin at day 50 after HCT. N = 3.
As mentioned in the first paragraph of the “Results” section, WT-cGVHD recipients continued to have cutaneous GVHD, whereas IgHµγ1-transient-cGVHD recipients recovered from cutaneous GVHD by 60 days after HCT (Figure 1). Thus, we compared Th1 and Th17 tissue distribution in those recipients at day 60. In general, the percentage of Th1 cells in the spleen, PLNs, and skin in all 3 groups decreased between days 40 and 60 (supplemental Figure 5A-B). At day 60, the percentages of Th1 cells in the spleen and PLNs of WT-cGVHD recipients were not significantly different from those in IgHµγ1-transient-cGVHD recipients, although the percentages were higher in WT recipients than in IgHµγ1-no-cGVHD recipients (P < .05; supplemental Figure 5B). At day 60, the percentage of Th1 cells in the skin was similar in all 3 groups (supplemental Figure 5B). This result suggests that Th1 cells may not play an important role in the persistence of cGVHD at 60 days after HCT.
On the other hand, tissue distribution of Th17 and Th1 cells differed from each other at 60 days after HCT. In the spleen, all 3 groups showed no significant increase in the percentage of Th17 cells between days 40 and 60, that is, ∼2%. However, the percentages of Th17 cells in the PLNs and skin of WT-cGVHD recipients (∼20%) were markedly higher than in IgHµγ1-no-cGVHD recipients (∼3%) (P < .01, Figure 4B). The percentage of Th17 cells in the skin of IgHµγ1-transient-cGVHD recipients was ∼3% and did not differ from the percentage in IgHµγ1-no-cGVHD recipients (Figure 4B).These results indicate that persistence of cutaneous cGVHD in the WT-cGVHD recipients is associated with infiltration of Th17 cells in the skin. The results also suggest that this process is augmented directly or indirectly by antibody secretion from donor B cells.
Antibody-producing donor B cells associate with increased release of CCL20 in the skin of cGVHD recipients
Because Th17 cells usually express CC chemokine receptor 6 (CCR6), which binds to CC chemokine ligand 20 (CCL20) and regulates Th17 tissue infiltration,36,37 we evaluated the expression of CCR6 by Th17 cells in the PLN and the expression of CCL20 in the skin of WT-cGVHD, IgHµγ1-no-cGVHD, and IgHµγ1-transient-cGVHD recipients at day 40 after HCT. The percentages of CCR6+CD4+TCRβ+ cells in the PLNs from the 3 groups followed the pattern observed with IL17+CD4+TCRβ+ cells in PLNs at day 40 (Figure 4A,C). Most of the Th17 cells were CCR6+ (Figure 4C), and WT-cGVHD recipients had markedly higher percentages of CCR6+IL17+CD4+ T cells, as compared with the other 2 groups (P < .01, Figure 4C bottom row). At day 40, all 3 groups expressed low levels of CCL20 mRNA in the skin. By day 60, skin from WT-cGVHD recipients expressed markedly higher levels of CCL20 mRNA as compared with the other 2 groups (P < .01, Figure 4D). The increased expression of CCL20 mRNA in the skin was associated with increased Th17 infiltration in the skin tissue of WT-cGVHD recipients (Figure 4B,D). These results suggest that donor B-cell antibodies directly or indirectly augment release of CCL20 in the skin of mice with cGVHD.
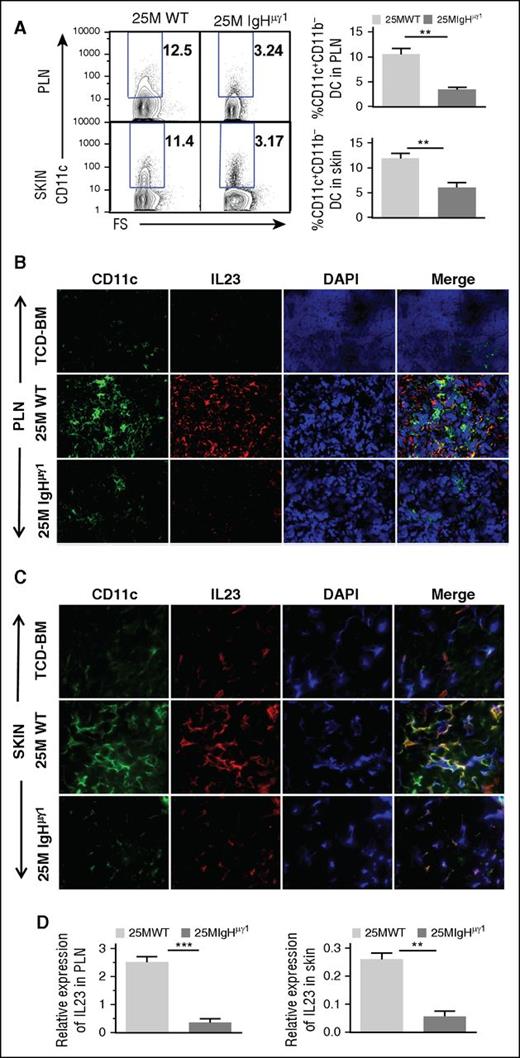
Antibody-producing donor B cells associate with increased DC secretion of IL-23 in PLNs and skin
Although few Th17 cells were present in the skin of WT-cGVHD recipients on day 40 (Figure 4A), the percentages of Th17 cells in the skin increased to ∼11% by day 50 (Figure 4E). Because dendritic cell (DC)–derived IL-23 plays an important role in augmenting expansion of Th17 cells,38 we evaluated the impact of antibodies from donor B cells on DC secretion of IL-23 in PLNs and skin. At day 40, the percentages of CD11b−CD11c+ DCs in the PLNs and skin of WT-cGVHD recipient were more than twofold higher than in IgHµγ1-no-cGVHD recipients (P < .01; Figure 5A). Histoimmunofluorescent staining also showed higher numbers of CD11c+ cells in the PLNs and skin of WT-cGVHD recipients, and the DCs appeared to have higher staining intensity of IL-23, as compared with IgHµγ1-no-cGVHD recipients (Figure 5B-C). Consistently, IL-23 mRNA expression in the PLN and skin of WT-cGVHD recipients was significantly higher than in IgHµγ1-no-cGVHD recipients (P < .01, Figure 5D). These results suggest that antibodies from donor B cells directly or indirectly induce IL-23 secretion by DCs in the PLNs and skin.
Antibody-producing donor B cells associate with increased DC secretion of IL-23 in PLNs and skin. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Day 40 after HCT, percentage of CD11b−CD11c+ DC in PLNs and skin. Representative patterns and means ± SE of the percentage of CD11b−CD11c+ cells in PLNs and skin are shown. N = 4 from at least 2 replicate experiments. (B-C) Frozen PLN (B) and skin (C) sections from BALB/C recipients given TCD-BM and WT or IgHμγ1 CD25−-SPL cells were stained with anti-CD11c (green), anti-IL23 (red), DAPI (blue, nucleus) 40 days after HCT. Photomicrographs (original magnification, ×400 for PLN; original magnification, ×600 for SKIN) representative of 4 replicate experiments are shown. (D) Means ± SE of IL-23 mRNA expression by PLNs and skin of recipients 40 days after HCT. Gene expression was normalized within each sample to the housekeeping gene GAPDH. N = 3 from 3 replicate experiments (**P < .01, ***P < .001).
Antibody-producing donor B cells associate with increased DC secretion of IL-23 in PLNs and skin. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Day 40 after HCT, percentage of CD11b−CD11c+ DC in PLNs and skin. Representative patterns and means ± SE of the percentage of CD11b−CD11c+ cells in PLNs and skin are shown. N = 4 from at least 2 replicate experiments. (B-C) Frozen PLN (B) and skin (C) sections from BALB/C recipients given TCD-BM and WT or IgHμγ1 CD25−-SPL cells were stained with anti-CD11c (green), anti-IL23 (red), DAPI (blue, nucleus) 40 days after HCT. Photomicrographs (original magnification, ×400 for PLN; original magnification, ×600 for SKIN) representative of 4 replicate experiments are shown. (D) Means ± SE of IL-23 mRNA expression by PLNs and skin of recipients 40 days after HCT. Gene expression was normalized within each sample to the housekeeping gene GAPDH. N = 3 from 3 replicate experiments (**P < .01, ***P < .001).
Injections of IgG-containing sera from WT-cGVHD recipients lead to tissue deposition of IgG and persistence of cGVHD in IgHµγ1-transient-cGVHD recipients
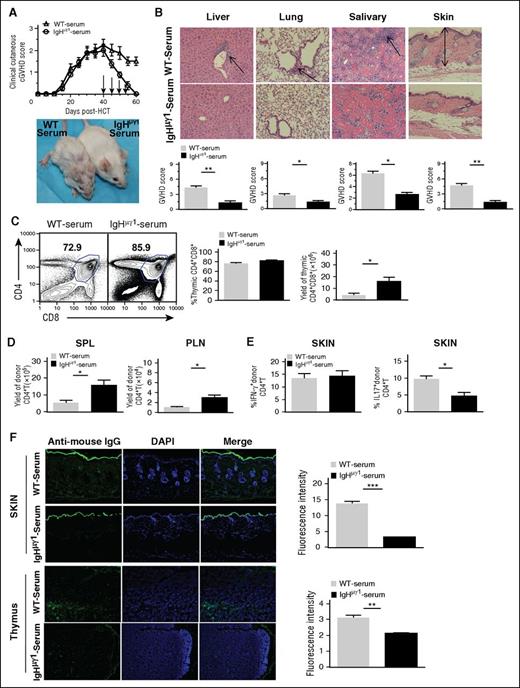
To directly test the contribution of antibodies from donor B cells in the persistence of cGVHD, we treated the IgHµγ1-transient-cGVHD recipients with injections of IgG-containing sera from WT-cGVHD recipients or from IgG-deficient sera from IgHµγ1-no-cGVHD recipients. Sera were injected at 40, 45, 50, and 55 days after HCT with 250 µL per mouse per injection. The recipients were monitored for clinical cutaneous cGVHD and compared by histopathology and skin Th17 infiltration as described in Figures 1 and 4. The clinical cutaneous cGVHD in the recipients injected with IgHµγ1-no-cGVHD sera gradually disappeared by 60 days after HCT (Figure 6A). In contrast, the clinical cutaneous cGVHD in the recipients injected with WT-cGVHD sera persisted for >60 days after HCT (P < .05, Figure 6A). Recipients with persistent cGVHD also showed (1) tissue damage in the liver, lung, salivary gland, and skin, as indicated by histopathology (P < .05, Figure 6B); (2) thymic damage as indicated by reduction of CD4+CD8+ thymocyte yield (P < .05, Figure 6C) and reduction of CD4+ T cells in the spleen and PLNs (P < .05, Figure 6D); (3) significant increase in the percentage of Th17 (P < .05) but not Th1 cells in the skin (Figure 6E); (4) significant increase in IgG deposition in the skin and thymus (P < .01, Figure 6F). These results indicate that IgG antibodies in WT-cGVHD recipients augment Th17 infiltration in the skin and enable development and persistence of cutaneous sclerosis.
Injections of IgG-containing sera from WT-cGVHD recipients lead to tissue deposition of IgG and persistence of cGVHD in IgHµγ1-transient-cGVHD recipients. BALB/c recipients were lethally irradiated (850 cGy, TBI) and given CD25-depleted splenocytes (75 × 106) and TCD-BM (2.5 × 106) cells from IgHμγ1 DBA/2 donors to set up IgHµγ1-transient-cGVHD recipients. Sera were harvested from WT-cGVHD or IgHµγ1-no-cGVHD recipients at 30 to 45 days after HCT. Serum aliquots (250 µL) were injected IV into IgHµγ1-transient-cGVHD recipients at 40, 45, 50, and 55 days after HCT. Recipients were monitored for cGVHD development for up to 60 days after HCT. N = 8 from 2 replicate experiments. (A) Clinical cutaneous GVHD scores. A representative photograph taken at day 60 is shown. (B) Histopathology of the GVHD target tissues liver, lung, salivary gland, and skin was evaluated. A representative photomicrograph (original magnification, ×200); means ± SE of histopathology scores from 6 recipients are shown. Arrows indicate infiltration in the liver, lymphocytic bronchiolitis, infiltration and loss of ductal structure in the salivary gland, hyperplasia in the epidermis, expansion of dermis and loss of subcutaneous fat. (C) Representative flow cytometry patterns of CD4+CD8+ double-positive thymocytes, and means ± SE of percentages and yields of double-positive thymocytes are shown. N = 4. (D) Yield of donor CD5.1+TCRβ+CD4+ T in the spleen and PLNs (means ± SE, N = 4). (E) Percentages of donor CD5.1+IFN-γ+CD4+ and CD5.1+IL-17+CD4+ T cells (means ± SE, N = 4-6). (F) Histoimmunofluorescent staining of mouse IgG (green) and DAPI (blue, nucleus) in thymus and skin sections. A representative photomicrograph (original magnification, ×100) and means ± SE (N = 4) of fluorescent intensity are shown (* P < .05, ** P < .01, ***P < .001).
Injections of IgG-containing sera from WT-cGVHD recipients lead to tissue deposition of IgG and persistence of cGVHD in IgHµγ1-transient-cGVHD recipients. BALB/c recipients were lethally irradiated (850 cGy, TBI) and given CD25-depleted splenocytes (75 × 106) and TCD-BM (2.5 × 106) cells from IgHμγ1 DBA/2 donors to set up IgHµγ1-transient-cGVHD recipients. Sera were harvested from WT-cGVHD or IgHµγ1-no-cGVHD recipients at 30 to 45 days after HCT. Serum aliquots (250 µL) were injected IV into IgHµγ1-transient-cGVHD recipients at 40, 45, 50, and 55 days after HCT. Recipients were monitored for cGVHD development for up to 60 days after HCT. N = 8 from 2 replicate experiments. (A) Clinical cutaneous GVHD scores. A representative photograph taken at day 60 is shown. (B) Histopathology of the GVHD target tissues liver, lung, salivary gland, and skin was evaluated. A representative photomicrograph (original magnification, ×200); means ± SE of histopathology scores from 6 recipients are shown. Arrows indicate infiltration in the liver, lymphocytic bronchiolitis, infiltration and loss of ductal structure in the salivary gland, hyperplasia in the epidermis, expansion of dermis and loss of subcutaneous fat. (C) Representative flow cytometry patterns of CD4+CD8+ double-positive thymocytes, and means ± SE of percentages and yields of double-positive thymocytes are shown. N = 4. (D) Yield of donor CD5.1+TCRβ+CD4+ T in the spleen and PLNs (means ± SE, N = 4). (E) Percentages of donor CD5.1+IFN-γ+CD4+ and CD5.1+IL-17+CD4+ T cells (means ± SE, N = 4-6). (F) Histoimmunofluorescent staining of mouse IgG (green) and DAPI (blue, nucleus) in thymus and skin sections. A representative photomicrograph (original magnification, ×100) and means ± SE (N = 4) of fluorescent intensity are shown (* P < .05, ** P < .01, ***P < .001).
Discussion
Donor B cells in cGVHD recipients are aberrantly activated10,39 and are proposed to play important roles in mediating cGVHD pathogenesis via their antigen-presenting and antibody-producing functions as well as their loss of regulatory function.11 In the current studies, we have added novel insights into how antibodies from donor B cells contribute to cGVHD pathogenesis. We have demonstrated that antibodies from donor B cells augment damage in the thymus, spleen, and lymph nodes, increase Th17 infiltration in the skin, and perpetuate cutaneous cGVHD in a murine model of MHC-matched HCT.
We observed that transplants with donor B cells that can secrete antibodies resulted in IgG antibody deposition in the thymus, causing severe damage and loss of mTECs. In contrast, transplants with B cells that cannot secrete IgG antibodies allowed for rapid recovery of total thymocyte numbers, percentages of CD4+CD8+ thymocytes, and percentages of mTECs to levels observed in TCD-BM controls. Injection of IgG-containing sera from WT-cGVHD recipients but not IgG-deficient sera from IgHµγ1-no-GVHD recipients led to IgG deposition in the thymus and reduction of thymocyte regeneration. These data identify a previously unidentified critical role for antibody deposition in the persistence of thymic dysfunction after the onset of cGVHD. Although CD4+ T cells initially cause self-limited damage through cellular immune mechanisms,40 CD4+ T-cell interaction with B cells and their production of IgG are required to perpetuate the damage.41,42
It has been proposed that lymphopenia in cGVHD recipients results from damage to lymphoid niches.43 Follicular DCs and lymphotoxin-producing stromal cells represent important lymphoid niches.44 Our observation that antibodies from donor B cells contribute to destruction of B-cell follicles and GCs as well as follicular DCs (data not shown) suggest that in addition to causing damage in the thymus, antibodies from donor B cells contribute to lymphopenia by damaging lymphoid niches in cGVHD recipients.
Our observations differ from those in other reports showing that GCs are enlarged in the lymphoid tissues of cGVHD recipients and that GC formation is required for persistence of cGVHD in murine models.19,25 The clinical relevance of these models is not yet clear. Human allogeneic HCT recipients recover production of IgG1 but not IgG2 or IgA or IgG somatic hypermuation within the first year after transplantation.45,46 These results suggest that GC formation or somatic hypermutation does not recover in humans until at least 1 year after allogeneic HCT, but cGVHD typically begins within the first year after HCT. The findings from our murine models consistently suggest that the pathogenesis of cGVHD does not require GC-dependent mechanisms. Further studies are needed to determine the extent to which various murine models reflect the pathogenesis and characteristics of cGVHD in different patient groups.
We previously reported that donor B cells in cGVHD augment pathogenic CD4+ T-cell expansion via their antigen-presenting function.9,15 We now provide an additional mechanism explaining how antibodies from donor B cells contribute to the development and perpetuation of cGVHD. The following evidence supports our conclusion that antibodies from donor B cells augment Th17 tissue infiltration and perpetuate cutaneous cGVHD. (1) High-level Th17 infiltration was observed in the PLNs of both WT-cGVHD and IgHµγ1-transient cGVHD recipients, but high-level Th17 infiltration in the skin was observed only in WT-cGVHD recipients. (2) Increased Th17 infiltration in the skin of WT-cGVHD recipients was associated with markedly increased tissue expression of CCL20 that attracts Th17 cells36,37 and increased DC expression of IL-23 that augments expansion of Th17 cells.38 (3) Injections of IgG-containing sera from WT-cGVHD recipients but not IgG-deficient sera from IgHµγ1-no-cGVHD recipients led to IgG deposition in the skin, increased infiltration of Th17 cells in the skin, and perpetuation of cutaneous cGVHD in IgHµγ1-transient-cGVHD recipients.
In conclusion, although donor B cells have a role in antigen presentation, antibody production by donor B cells also plays an important role in the pathogenesis of cGVHD. Blocking the signaling pathways mediated by autoantibodies in cGVHD recipients may still represent novel approaches for ameliorating cGVHD. Binding of agonistic autoantibodies to platelet-derived growth factor receptor can cause cutaneous fibrosis in patients with scleroderma or cGVHD,47,48 although clinical trials testing the use of tyrosine kinase inhibitors to block the downstream signaling pathway mediated through platelet-derived growth factor receptor have not produced consistent results in patients with cGVHD.49-53 The differences among these trials may be explained by variation in the spectrum of pathogenic autoantibodies or by antibody-independent mechanisms. Further studies will be needed to identify the mechanisms that account for antibody-mediated thymic and lymphoid tissue damage and cutaneous sclerosis in cGVHD. Possible mechanisms include binding to agonistic receptors that lead to sclerosis, complement-mediated damage, antibody-dependent cell-mediated toxicity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Arthur Riggs for providing bridge fund to support this project. The authors thank Lucy Brown and her staff at the City of Hope (COH) Flow Cytometry Facility, Sofia Loera and her staff at COH Anatomic Pathology Core, and Dr Richard Ermel and his staff at COH Animal Research Center for providing excellence service.
This work was supported by National Institutes of Health grants from the National Institute of Allergy and Infectious Diseases (R01-AI066008 and R56-AI066008) and the National Cancer Institute (P30CA33572) and the Nesvig Lymphoma Foundation (D.Z.) and by the National Natural Science Foundation of China grants NSFC 81270647 and 81470349 (Q.L.).
Authorship
Contribution: H.J. designed and performed research as well as prepared the manuscript; X.N., R.D., and Q.S. performed experiments; J.Y., M.Z. and K.C. assisted in experiments; P.J.M. critically reviewed and edited the manuscript; S.F. supported research and reviewed the manuscript; Q.L. is H.J.’s PhD advisor; and D.Z. designed and supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Defu Zeng, Departments of Diabetes Research and Hematology/Hematopoietic Cell Transplantation, Beckman Research Institute of City of Hope, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: dzeng@coh.org; or Qifa Liu, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China; e-mail: liuqifa628@163.com.

![Figure 3. Antibody-producing donor B cells associate with thymus damage in cGVHD recipients. HCT was carried out with BALB/c recipients as described in Figure 1. (A) Analysis of CD4+CD8+ double-positive thymocytes at 40 and 60 days after HCT. Staining profiles representative of 4 replicate experiments are shown, Means ± SE are displayed for percentage and yield of double-positive thymocytes at 40 and 60 days after HCT. (B) On days 40 and 60 after HCT, BALB/C recipient thymus cell suspensions were measured for the percentage of mTECs. Gated thymic epithelial cells (TECs) are shown as UEA I (medullary TEC [mTEC] marker) vs Ly51 (cortical TEC marker). A flow cytometry pattern representative of 4 replicate experiments is shown. Means ± SE (N = 4) of the percentage of mTECs are shown. (C) On day 40 after HCT, thymus sections were analyzed by histoimmunofluorescent staining for cytokeratin 8 (red, cortical epithelial cells) and UEA-I (green, mTEC). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown. (D) On day 40 after HCT, thymus sections were evaluated by histoimmunofluorescent staining with anti-mouse IgG-FITC (green) and 4,6 diamidino-2-phenylindole (DAPI) (blue, nucleus). Photomicrographs (original magnification, ×100) representative of 4 replicate experiments are shown (**P < .01, ***P < .001, ****P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/18/10.1182_blood-2015-09-668145/4/m_2249f3.jpeg?Expires=1769946202&Signature=1ys2MRRtQTQgSh7FwH8bvp1ZLGlIAcbRb4VmZEfk~oJy2S1Ia4yD8w0QiSfxH38msl-FSQqmje9kdxOfXUIZi8JUROqEKj4g8bLYfwEyq60Nal7K5avhisr-beeoj0CKm9y1kkN~J1nrmpzQEzwkQfv5tsOgxS21l3gZzhL3SChCzYeIkpma7W-0sBBbaEnrbfVrvqMdkwM~9SW6umfK3nWfioYWrMjyJPWqkZDjSdTynLkLGSqfgp6GUm5jfngPQqU2OvriFF-icY9G2yeCZuF43EAUwCwKwMoYRqQhjWxbs-Qn4JoNNcQyEdwHkaMoJdjXwymmXvqA9xVOg~k0VA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal