In this issue of Blood, Zhou et al have identified synergistic in vitro and in vivo anti–acute myeloid leukemia (AML) activity of the lysine neddylation inhibitor pevonedistat and pan–histone deacetylase (HDAC) inhibitor (pan-HDACI) belinostat and examine the mechanisms responsible for this synergistic activity.1
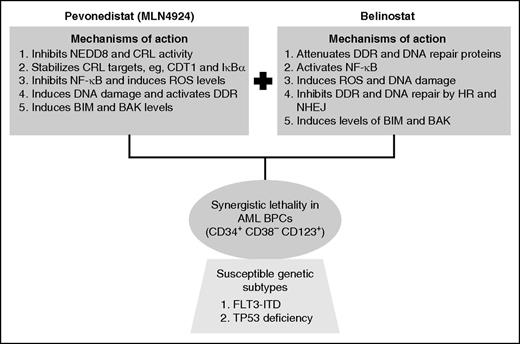
Mechanistic interactions creating synergy between pevonedistat and belinostat against AML blast progenitor cells (BPCs).
Mechanistic interactions creating synergy between pevonedistat and belinostat against AML blast progenitor cells (BPCs).
Protein lysine neddylation is a posttranslational modification in which the ubiquitin-like molecule NEDD8 (neural precursor cell expressed, developmentally downregulated 8) is covalently linked to several cellular proteins, including the E3 ubiquitin ligases known as cullin-ring E3 ligases (CRLs).2 Neddylation activates CRLs, which in turn ubiquitylate and degrade, via the proteasome, a variety of their substrate-protein targets, including CDT1 (chromatin-licensing and DNA replication factor), CDKN1B (cyclin-dependent kinase inhibitor 1B) (p27), IκBα (nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor, α), and NRF2 (nuclear factor, erythroid 2–like 2).2,3 The first step leading to CRL neddylation is catalyzed by the NEDD8 activating enzyme (NAE), and pevonedistat (MLN4924) has been developed as an irreversible inhibitor of the NAE.2,4 Treatment of cells with pevonedistat inhibits neddylation and CRL activity, leading to stabilization and accumulation of the CRL substrate-proteins noted before. This has been shown to cause nuclear factor–κB (NF-κB) inhibition, reactive oxygen species (ROS) accumulation, DNA re-replication, DNA damage, as well as in vitro and in vivo lethality in AML cells.4,5 Notably, treatment with pevonedistat simultaneously induces DNA damage and DNA damage response (DDR) but compromises DNA repair, thereby sensitizing transformed cells to DNA-damaging agents.3,4,6 Based on its promising preclinical, in vitro, and in vivo anti-AML activity, phase 1 clinical trials of pevonedistat were conducted in patients with relapsed/refractory AML or myelodysplastic syndrome.7 Although hepatotoxicity was dose-limiting, complete and partial remissions were observed at or below the maximal tolerated dose of pevonedistat.7 Collectively, these findings underscored the potential of developing rational combinations of pevonedistat with agents that would increase its anti-AML efficacy and exert synergistic lethality against AML. In this issue of Blood, Zhou et al chose the class I and II HDACI belinostat to fulfill this role.1 HDACs are commonly overexpressed in cancer and AML cells.8 HDACs are also recruited by oncogenic fusion proteins in AML (eg, AML1-ETO and PML-RARα) to repress target genes involved in differentiation and apoptosis of AML cells.8 Over the past decade, several preclinical studies have shown that pan–HDACIs, such as belinostat, induce ROS, DNA damage, differentiation, and apoptosis in AML cells.8 However, despite their promising preclinical anti-AML efficacy, single-agent clinical activity of HDACI in AML has been quite modest and disappointing.8,9 Yet, HDACI did display significant clinical activity against cutaneous T-cell or peripheral T-cell lymphoma and are therefore approved as a therapy for these clinical entities.8
In the studies reported in this issue of Blood, Zhou et al examined the preclinical activity of a combination of pevonedistat and belinostat.1 They demonstrated that, compared with treatment with each agent alone, combined therapy with pevonedistat and belinostat exerts in vitro synergistic lethality against a variety of cultured AML cell types with diverse genetic backgrounds, including the presence of FLT3-ITD and MLL-AF4 (as in MV4-11 cells), as well as the deficiency of wild-type TP53 (see figure). The combination was also synergistically lethal against patient-derived primary AML cells. Furthermore, cotreatment with pevonedistat and belinostat significantly improved the survival of immune-depleted mice engrafted with MV4-11 cells. What might be the basis of the potent anti-AML activity of this combination? The authors demonstrate multiple mechanisms that may be involved. First, whereas pan-HDACI, such as belinostat, activates prosurvival activity of NF-κB, cotreatment with pevonedistat inhibits NF-κB, thereby potentiating HDACI-mediated lethality in AML cells. Second, although pevonedistat treatment promotes DNA damage, activates DDR, and induces activity of the homologous recombination (HR) repair–related proteins, cotreatment with belinostat attenuated the levels of proteins involved in the DDR and DNA repair through HR and nonhomologous end-joining mechanisms. Consistent with this, belinostat treatment inhibited the DNA repair foci in the nucleus, thereby markedly increasing the single- and double-strand DNA damage and cell death. Third, although pevonedistat stabilized the DNA re-replication licensing factor CDT1 and activated the intra-S phase checkpoint, thereby promoting chromosome decondensation and elongation,2-4 cotreatment with belinostat led to chromosome pulverization and increased lethality. How do these mechanistic interactions upstream between combination partners markedly reduce the threshold for apoptosis? It was demonstrated that cotreatment with pevonedistat and belinostat is associated with induction of BH3 domain-only proteins BIM and NOXA, as well as the multidomain proapoptotic protein BAK. These alterations are likely to be the final trigger for the ensuing caspase-dependent AML cell death.4
It is noteworthy that, compared with the normal CD34+ progenitor cells, the combination of pevonedistat and belinostat was clearly more lethal against the AML blast progenitor cells, especially the leukemia-initiating cells (LICs) characterized by a CD34+ CD38– CD123+ phenotype.10 With respect to any novel targeted therapy, it is important to determine whether the effectiveness of the therapy is limited to specific genetic subtypes and/or molecular pathway dependencies, which would increase the chances for identifying patient populations that would benefit the most from the novel therapy. Therefore, it is also significant that the lethal effect after cotreatment with pevonedistat and belinostat were observed on LICs from AML of relatively diverse genetic backgrounds. However, whether the combination also undermined the functional capacity of the LICs to initiate AML in the relevant mouse models was not investigated.
Going forward, the promising preclinical anti-AML efficacy of the cotreatment with pevonedistat and belinostat demonstrated by Zhou et al, coupled with the documented single-agent clinical activity of pevonedistat in AML, creates a strong rationale to further evaluate the efficacy of the combination in a phase 2 trial in patients with AML. Importantly, to determine the predictors of response or resistance to the combination, studies involving the genetic profiling by whole-exome DNA- and RNA-sequencing, as well as evaluation of selected biomarkers of the on-target effects of pevonedistat and belinostat in the AML cell samples, must be incorporated in the trial. This would more rationally guide the future clinical development of the combination in the therapy of AML.
Conflict-of-interest disclosure: The authors declare no competing financial interests.