In this issue of Blood, Böll et al report on the toxicity of 2 vs 4 cycles of bleomycin-containing chemotherapy in older, early stage, favorable patients with Hodgkin lymphoma (HL) and show that 2 cycles, but no more, of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is well tolerated in this patient population.1
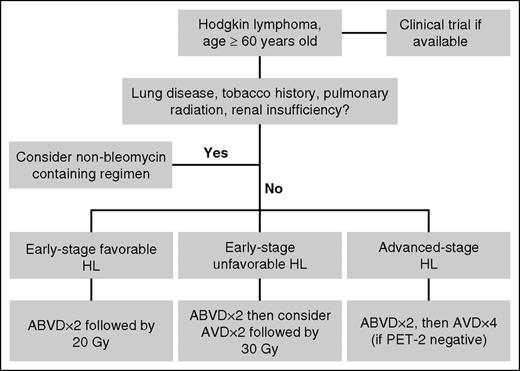
Potential algorithm for older HL patients. Clinical trials should be considered for all patients when available. For patients who lack risk factors for bleomycin lung toxicity, consider using no more than 2 cycles of bleomycin-containing chemotherapy. PET, positron emission tomography.
Potential algorithm for older HL patients. Clinical trials should be considered for all patients when available. For patients who lack risk factors for bleomycin lung toxicity, consider using no more than 2 cycles of bleomycin-containing chemotherapy. PET, positron emission tomography.
Compared to younger patients, patients over 60 years of age with HL have significantly inferior outcomes. Older HL patients are not only more likely to have risk factors associated with poor prognosis, such as B symptoms, mixed cellularity type, or poor performance status, but a major reason for their poor outcomes is reduced tolerability of treatment.2,3 In the North American Intergroup Study, overall treatment-related mortality was 9% for patients over 60 compared with 0.3% for younger patients. Furthermore, bleomycin lung toxicity was observed in 43% of older patients treated with ABVD, which is significantly higher than expected for younger patients.3 The German Hodgkin Study Group (GHSG) reported a similar experience in the HD10 and HD11 studies, in which patients 60 years and older receiving 4 cycles of ABVD experienced substantial dose reductions, delays, toxicity, and treatment-related toxicity.4 As a result, treatment approaches for older HL patients vary. Some fit patients are treated with standard regimens designed for younger patients, whereas others receive non–bleomycin-containing regimens, such as AVD, chlorambucil, vinblastine sulfate, procarbazine, and prednisolone, or prednisone, vinblastine sulfate, adriamycin, and gemcitabine5,6 ; however, the optimal treatment of this group is not known. It is apparent, based upon the results of the GHSG HD13 study, that complete elimination of bleomycin, even for the most favorable patients, leads to inferior outcomes; therefore, defining the optimal number of bleomycin-containing chemotherapy cycles for older patients may help improve outcomes.7
In their analysis, Böll et al1 focused on older patients enrolled in the GHSG HD10 and HD13 studies. Both studies enrolled only patients with favorable disease based upon the GHSG risk groups. In the HD10 study, patients were randomized to receive either 2 or 4 cycles of ABVD followed by 20 or 30 Gy-involved field radiation.8 In the HD13 study, patients were randomized to receive 2 cycles of ABVD, AVD, ABV, or AV followed by 30 Gy-involved field radiation.7 Between the 2 studies, 287 patients aged 60 or older received 2 cycles of ABVD (n = 137), AVD (n = 82), or 4 cycles of ABVD (n = 68). This allowed for direct comparison of toxicity of 0, 2, and 4 cycles of bleomycin-containing chemotherapy for older patients.
The authors found that 2 cycles of AVD and ABVD were fairly similar with respect to early termination of treatment (2% and 4%, respectively) and frequency of grade 3-4 adverse events (40% and 39%, respectively). In contrast, 4 cycles of ABVD was associated with a considerably higher rate of early treatment termination (18%) and grade 3-4 toxicity (65%). With regard to bleomycin-induced lung toxicity, 0% and 1.5% cases were reported among patients receiving 2 cycles of AVD and ABVD, respectively, whereas 7 cases (10%) were reported among patients receiving 4 cycles ABVD, 3 of which were fatal.
In applying this data to the care of older patients with HL, it is important to note that most patients included in this analysis had good performance status (most with Eastern Cooperative Oncology Group grade 0 or 1) and were fairly young in age (median age, 64-66 years). Furthermore, as mentioned by the authors, comprehensive geriatric assessments were not included in the HD10 or HD13 studies, therefore the impact of factors such as functional status, fall risk, and social support on treatment toxicity within these studies is not known. In the retrospective analysis by Evens et al, age 70 (or greater) and loss of activities of daily living were the most important adverse prognostic factors in this patient population.5 Ongoing and future prospective studies will determine whether these factors predict for treatment toxicity and need to be considered when deciding upon treatment courses. Current studies for elderly patients incorporating novel agents for HL, such as the ongoing study with sequential brentuximab vedotin and AVD (clinicaltrials.gov #NCT01476410) may obviate the need for bleomycin in this population. For now, though, this analysis from the GHSG provides us with a framework for using bleomycin in older patients (see figure). At the very least, we should aim to use no more than 2 cycles of chemotherapy with bleomycin for older HL patients. In early-stage disease, this is accomplished by using radiation consolidation to shorten the course of chemotherapy. In advanced-stage disease, it is appropriate to treat as per the response-adjusted therapy for HL study, in which patients with PET-2–negative scans had bleomycin removed after 2 cycles of ABVD with no adverse impact on tumor control.9 As the authors mention, known risk factors for bleomycin toxicity, such as underlying lung disease, renal insufficiency, pulmonary radiation, and the tobacco history could not be assessed in their analysis due to the small numbers; however, the use of bleomycin for patients with these comorbities should likely be avoided altogether. Ongoing and future prospective studies, which incorporate comprehensive geriatric assessments and novel HL agents, are likely to facilitate the development of risk-adapted treatment approaches for older patients with HL and hopefully improve outcomes for this group.
Conflict-of-interest disclosure: A.M. received research funding from Seattle Genetics.