Key Points
Platelet-derived microparticles inhibit IL-17 and IFN-γ production by Tregs and stimulate Treg stability in an inflammatory environment.
Platelet-derived microparticles inhibit Treg plasticity in a P-selectin– and partially CXCR3-dependent manner.
Abstract
Self-tolerance and immune homeostasis are orchestrated by FOXP3+ regulatory T cells (Tregs). Recent data have revealed that upon stimulation, Tregs may exhibit plasticity toward a proinflammatory phenotype, producing interleukin 17 (IL-17) and/or interferon γ (IFN-γ). Such deregulation of Tregs may contribute to the perpetuation of inflammatory processes, including graft-versus-host disease. Thus, it is important to identify immunomodulatory factors influencing Treg stability. Platelet-derived microparticles (PMPs) are involved in hemostasis and vascular health and have recently been shown to be intimately involved in (pathogenic) immune responses. Therefore, we investigated whether PMPs have the ability to affect Treg plasticity. PMPs were cocultured with healthy donor peripheral blood–derived Tregs that were stimulated with anti-CD3/CD28 monoclonal antibodies in the presence of IL-2, IL-15, and IL-1β. PMPs prevented the differentiation of peripheral blood–derived Tregs into IL-17– and IFN-γ–producing cells, even in the presence of the IL-17–driving proinflammatory cytokine IL-1β. The mechanism of action by which PMPs prevent Treg plasticity consisted of rapid and selective P-selectin–dependent binding of PMPs to a CCR6+HLA-DR+ memory-like Treg subset and their ability to inhibit Treg proliferation, in part through CXCR3 engagement. The findings that ∼8% of Tregs in the circulation of healthy individuals are CD41+P-selectin+ and that distinct binding of patient plasma PMPs to Tregs was observed support in vivo relevance. These findings open the exciting possibility that PMPs actively regulate the immune response at sites of (vascular) inflammation, where they are known to accumulate and interact with leukocytes, consolidating the (vascular) healing process.
Introduction
Although platelets are well known to be critical for hemostasis, it is becoming increasingly clear that platelets can actively modulate immune responses.1,2 Upon activation, platelets release chemokines, cytokines,1 and plasma membrane platelet-derived microparticles (PMPs) into the circulation.3 PMPs are in the submicron range, are involved in hemostasis, and expose the lipid phosphatidylserine and molecules specific for platelets.3 They have the ability to transfer membrane receptors4 and microRNA5 to other cells and play a role in the regulation of immunity.3,6
Forkhead box P3 (FOXP3)+ regulatory T cells (Tregs) are fundamental for immune homeostasis. Naturally occurring FOXP3+, CD25high, and CD127low/− Tregs comprise ∼5% of the human peripheral blood CD4+ T cells.7 FOXP3 gene hyperdemethylation is key to high and constitutive FOXP3 expression, which in turn is crucial for Treg suppressor function8 and Treg stability.7 We and others have shown that upon activation, CD4+CD25highFOXP3+ Tregs can lose FOXP3 and differentiate into interleukin 17 (IL-17)– and interferon γ (IFN-γ)–producing cells.9-13 Although proinflammatory cytokine–producing Tregs may reveal reduced suppressive capacity, a subset of IL-17–producing cells was shown to retain both FOXP3 expression and suppressive function.9,11,14,15 Of note, Wang et al showed that in rheumatoid arthritis patients, peripheral blood IL-17–producing Tregs were suppressive, whereas their synovial fluid counterparts lost suppressive capacity.15 The notion that this plasticity might have clinical implications was fueled by a mouse study revealing that cytokine-producing Tregs induced autoimmunity.16 In humans, increased numbers of proinflammatory cytokine–producing Tregs were found in patients with multiple sclerosis,17 Crohn disease,18 and psoriasis,19 where we identified IL-17–producing Tregs in the inflamed skin.19
The ability of PMPs to influence cells of the immune system under inflammatory conditions that can also affect Treg phenotype led us to investigate the direct effect of PMPs in Tregs. Here, we show that PMPs inhibit the differentiation of highly purified human peripheral blood Tregs into IL-17– and IFN-γ–producing cells. The binding of PMPs to Tregs, and the subsequent inhibition of Treg differentiation, appeared to be P-selectin and partly CXCR3 dependent. Prior to and during Treg activation and subsequent differentiation, PMPs selectively bound a specific subset of memory-like Tregs expressing CCR6 and HLA-DR, known to bring forth IL-17–producing cells.10,20 In the circulation, we found that ∼8% of Tregs are CD41 and P-selectin double positive. The finding that PMPs affect Treg proliferation provides a possible mechanism by which PMP binding to the memory-like Treg subset affects their IL-17–producing capacity.
These findings open the exciting possibility that PMPs actively regulate the immune response at sites of (vascular) injury, where PMPs are known to accumulate.21
Methods
HIV-infected adults, acute coronary syndrome patients, and healthy volunteers
Peripheral blood (EDTA) was obtained from patients and healthy volunteers.
HIV-infected adults on stable combination antiretroviral therapy with undetectable viral load (<50 copies/mL) and CD4 counts >300 cells/mm3 were eligible for enrollment. Exclusion criteria were the use of platelet inhibitors or an active concomitant infection (eg, hepatitis C or B). The study protocol was approved by the medical ethical committee Arnhem-Nijmegen (Radboud University Medical Center).
Patients with a diagnosis of acute coronary syndrome (ACS; ST-elevation myocardial infarction and non–ST-elevation myocardial infarction). Blood samples were collected 4 to 12 hours from the start of chest pain. Patients with evidence of infectious diseases, malignancies, hematologic or immunologic disorders, treatment with anti-inflammatory drugs other than aspirin, and an ejection fraction <40 were excluded. The study was approved by the local research ethics committee (St. George’s, University of London).
Both studies adhered to the Declaration of Helsinki. Signed informed consent was obtained from all patients and healthy volunteers.
PMP isolation, storage, and anti–P-selectin monoclonal antibody neutralization
Single donor platelet-rich plasma (PRP) was collected from 3 healthy donors by apheresis upon written informed consent according to the Declaration of Helsinki. Using a component collection system (MCS+; Haemonetics, Braintree, MA) in combination with acid citrate dextrose solution A, 300 × 109 leukoreduced (<1 × 106 leukocytes/U) platelets were collected in 300 mL plasma and stored in a lateral shaking device at 22°C ± 2°C for 7 days.
At day 7 of storage (the maximum storage period in The Netherlands), PMPs were isolated from the PRP product under sterile conditions using differential centrifugation as previously described.22 The PRP was centrifuged at 2000g for 15 minutes to remove platelets and cell debris. The supernatant was aliquoted, centrifuged for 45 minutes at 20 000g to pellet PMPs, and washed with 0.22 µm filtered calcium-free Ringer’s solution (125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 32 mM HEPES, 5 mM glucose, and 0.2% bovine serum albumin [pH 7.4]). The PMPs were resuspended in calcium-free Ringer’s solution, snap-frozen with liquid N2, and stored at −80°C. PMP purity/quantity was assessed using a Gallios flow cytometer (Beckman Coulter, Brea, CA) as described in supplemental Methods (available on the Blood Web site). PMP-associated P-selectin neutralization and washed platelet isolation, activation, and subsequent PMP generation are described in the supplemental Methods. Plasma-derived microparticle isolation is described in the supplemental Methods.
Cell isolation, culture, and anti-CXCR3 and anti-CD40 mAb neutralization
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation (Lymphoprep; Nycomed-Pharma AS, Oslo, Norway) of buffy coats obtained from healthy donors upon written informed consent according to the Declaration of Helsinki. CD4+ T cells were purified from PBMCs by magnetic bead sorting using CD4 MicroBeads (Miltenyi Biotec, Leiden, The Netherlands) according to the manufacturer’s instructions. Purified CD4+ T cells were labeled with anti-CD4–fluorescein isothiocyanate (FITC) (MT310; DAKO, Glostrup, Denmark) and anti-CD25-PE (MA251; BD Biosciences, Erembodegem, Belgium) monoclonal antibodies (mAbs), after which CD4+CD25high cells (Tregs) were isolated by high-purity (>99%) flow-cytometric cell sorting using an Aria cell sorter (Beckman Coulter).
Cells (2.5 × 104/well) were cultured in culture medium (RPMI-1640 with Glutamax supplemented with 0.02 mM pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin [Gibco, Paisley, United Kingdom], and 10% human pooled serum) at 37°C, 95% humidity, and 5% CO2 in 96-well round-bottom plates (Greiner, Frickenhausen, Germany). Anti-CD3/anti-CD28 mAb–coated beads (1:10; Invitrogen, Breda, The Netherlands) and recombinant human cytokines IL-2 (25 U/mL; Cetus, Amsterdam, The Netherlands), IL-15 (10 ng/mL), and IL-1β (50 ng/mL) (Biosource, Etten-Leur, The Netherlands) were added at the start of the cultures, in the presence or absence of PMPs. The suppression assay, transwell assay, and CD40 and CXCR3 neutralization are described in supplemental Methods.
Flow cytometry and antibodies
Cells were phenotypically characterized using the Navios flow cytometer (Beckman Coulter) and conjugated mAbs supplied in supplemental Methods. In order to study cell proliferation, Pacific Blue succinimidyl ester (Invitrogen) labeling (0.012 mg/mL) of Tregs prior to culture was performed. Intracellular analysis was performed after fixation and permeabilization using Fixation/Permeabilization reagent (eBioscience, Uithoorn, The Netherlands). Before intracellular cytokine measurements, the cells were stimulated for 4 hours with phorbol 12-myristate 13-acetate (PMA; 12.5 ng/mL) plus ionomycin (500 ng/mL) in the presence of Brefeldin A (5 µg/mL; Sigma-Aldrich, Zwijndrecht, The Netherlands). Peripheral blood staining is described in supplemental Methods.
All flow cytometry data were analyzed using Kaluza software (Beckman Coulter). Proliferation Platform of FlowJo 7.6 was employed to assess proliferation.
Confocal laser scanning microscopy
See supplemental Methods.
FOXP3 gene methylation
See supplemental Methods.
Statistics
Repeated-measures 1-way (plus Tukey’s post-test) or 2-way (plus Bonferroni post-test) ANOVAs were used for statistical analysis. The reported (2-sided) P values of < .05 were considered significant and are indicated with an asterisk (*). Data in the text are presented as mean ± standard deviation.
Results
PMPs inhibit CD25highFOXP3+ Treg differentiation into IL-17–producing cells
We reasoned that PMPs, with their arsenal of immune-mediating molecules,3 and their ability to interact with cells of the immune system,6,23 might affect the differentiation of Treg into IL-17–producing T cells.9
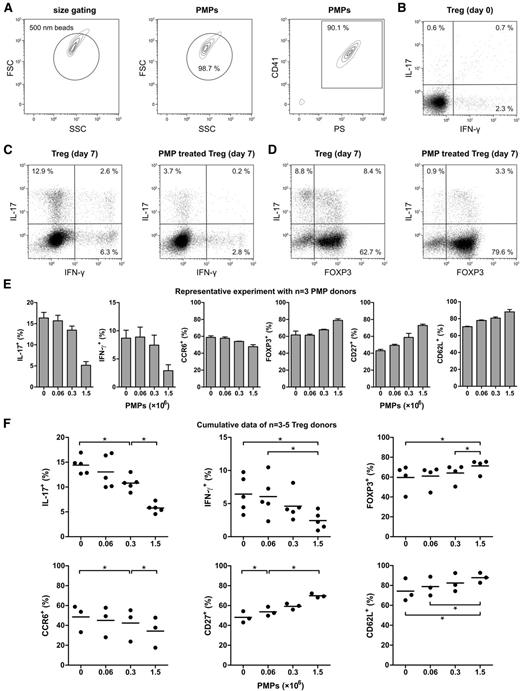
To obtain large amounts of PMPs, we used PMPs isolated from 7-day–stored platelet apheresis products of healthy donors, known to accumulate PMPs during storage.24 PMP purity, size, and number were assessed by flow cytometry, based on exposure of phosphatidylserine (PS) and CD41 (Figure 1A and supplemental Figure 1). Over 99% of all measured events were smaller than 1 µm in diameter, and more than 90% stained positive for CD41 and PS. Stored platelets and their PMPs were further characterized for exposure of PS, CD40, CD41, and activation markers P-selectin and CD63 (supplemental Figure 2).
PMPs inhibit Treg differentiation into IL-17–producing cells. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) CD41+PS+ PMPs <1 µm were purified from platelet apheresis units of healthy donors by differential centrifugation and analyzed by flow cytometry for phosphatidylserine (PS) and CD41 exposure as described in “Methods.” Size beads (500 nm) were used to set a size-exclusion gate, and fluorescent count beads (∼10 µm) were added for quantification. The contour plots are representative of 6 donors. (B) Representative experiment showing intracellular IL-17 and IFN-γ staining of the Treg at day 0. (C-D) Representative example of intracellular IFN-γ, IL-17, and FOXP3 expression by Tregs that were cultured for 7 days with and without 1.5 × 106 PMPs. (E) A representative experiment performed with Tregs from a single donor and increasing PMP concentrations from 3 different healthy donors. Surface CCR6, CD27, CD62L, and intracellular IL-17, IFN-γ, and FOXP3 staining of the Treg at day 7 of culture is shown. Error bars represent standard deviation (SD). (F) Cumulative data of different Treg donors (n = 3-5) showing surface CCR6, CD27, CD62L, and intracellular IL-17, IFN-γ, and FOXP3 staining of Tregs cultured for 7 days with increasing PMP concentrations. Each donor’s Tregs are shown as a dot representing the mean response of those Tregs to the three different donor’s PMPs. The mean is presented as bars. *P < .05. FSC, forward scatter; SSC, side scatter.
PMPs inhibit Treg differentiation into IL-17–producing cells. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) CD41+PS+ PMPs <1 µm were purified from platelet apheresis units of healthy donors by differential centrifugation and analyzed by flow cytometry for phosphatidylserine (PS) and CD41 exposure as described in “Methods.” Size beads (500 nm) were used to set a size-exclusion gate, and fluorescent count beads (∼10 µm) were added for quantification. The contour plots are representative of 6 donors. (B) Representative experiment showing intracellular IL-17 and IFN-γ staining of the Treg at day 0. (C-D) Representative example of intracellular IFN-γ, IL-17, and FOXP3 expression by Tregs that were cultured for 7 days with and without 1.5 × 106 PMPs. (E) A representative experiment performed with Tregs from a single donor and increasing PMP concentrations from 3 different healthy donors. Surface CCR6, CD27, CD62L, and intracellular IL-17, IFN-γ, and FOXP3 staining of the Treg at day 7 of culture is shown. Error bars represent standard deviation (SD). (F) Cumulative data of different Treg donors (n = 3-5) showing surface CCR6, CD27, CD62L, and intracellular IL-17, IFN-γ, and FOXP3 staining of Tregs cultured for 7 days with increasing PMP concentrations. Each donor’s Tregs are shown as a dot representing the mean response of those Tregs to the three different donor’s PMPs. The mean is presented as bars. *P < .05. FSC, forward scatter; SSC, side scatter.
PMPs were then cocultured with highly purified CD4+CD25highCD27+CD127low/−FOXP3+ Treg isolated from PBMCs by fluorescence-activated cell sorting (supplemental Figure 3). Typically, anti-CD3/CD28 mAb stimulation of this population in the presence of IL-2, IL-15, and IL-1β leads to a substantial number of IL-17–producing cells, with concomitant loss of FOXP3.9
The addition of PMPs to the freshly isolated Tregs at ratios of 2.4, 12, or 60 PMPs per cell inhibited the induction of IL-17 and IFN-γ production in a dose-dependent manner (Figure 1B-C,E-F). Similarly, the loss of FOXP3 expression was inhibited (Figure 1D-F), and a higher level of CpG demethylation in the Treg-specific demethylation region of the FOXP3 gene was detected (supplemental Figure 4). Interestingly, of the few cells that managed to acquire IL-17–producing capacity in the presence of PMPs, most still expressed FOXP3 (Figure 1D), suggesting that the differentiation process was partially hampered. Furthermore, PMP binding was higher in IL-17+FOXP3+ cells than in IL-17+FOXP3− cells (supplemental Figure 5). Previous studies have revealed that CD4+CD25+ Tregs expressing CD27 and/or CD62L constitute highly suppressive Treg subsets.25,26 Similar to the expression of FOXP3, loss of CD27 and CD62L was also prevented, whereas PMPs inhibited the percentage of cells that acquired the TH1727 and memory-associated28 chemokine receptor CCR6 during culture (Figure 1E-F). PMPs isolated from 2-day–stored platelet apheresis products also inhibited the generation of IL-17–producing cells (supplemental Figure 6), indicating that there are no major qualitative storage period–associated differences in this regard.
To examine whether the effects of PMPs on Treg differentiation are stored-platelet specific or can be attributed to other sources and causes of PMP production, we used freshly isolated washed platelets activated with thrombin receptor activator peptide 6 (TRAP) in the presence of calcium and shear stress for optimal PMP generation. The PMPs formed by these TRAP-activated washed platelets had similar effects on Treg differentiation as PMPs isolated from stored platelet units, including reduced induction of IL-17 and IFN-γ production (supplemental Figure 7). These data underscore the potential of both platelet apheresis unit–derived, as well as activated platelet-derived, PMPs to promote Treg stability. In addition, we also observed a reduction in IL-17 production when culturing Tregs in the presence of TRAP-activated washed platelets themselves (supplemental Figure 8).
P-selectin and CXCR3 mediate PMP inhibitory effects on Treg differentiation
Subsequently, the question arose which molecular component(s) of the PMPs were responsible for the inhibitory effect observed. Using a transwell setup with intracellular IL-17 expression as a readout (Figure 2A), we observed that Treg differentiation was contact dependent. This ruled out the possibility of cytokine scavenging or release by the PMPs significantly affecting Treg differentiation.
PMP inhibition of Treg differentiation is P-selectin dependent. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) Intracellular IL-17 expression in Tregs that were cocultured with PMPs as a standard mixed culture (control) or separated by a porous membrane (transwell). (B) The presence of the platelet-specific marker CD41 was assessed on the CD4+ Tregs after coculture with increasing PMP concentrations. Each donor’s Tregs are shown as a dot representing the mean response of those Tregs to the 3 different donors’ PMPs. The mean is presented as bars. *P < .05. (C) Representative contour plots of PMPs stained with anti–CD41-PE and anti–P-selectin-FITC or isotype mAbs. The contour plots are representative of 3 donors. (D) Treg expression of PSGL-1 and CD25 at day 0 of culture. (E) Surface CD41, CD27, and intracellular expression of IL-17 on Treg cocultured with untreated, isotype, or anti–P-selectin mAb-neutralized PMPs. The graphs are representative of 2 Treg donors. Mean and SD are shown. *P < .05. (F) Confocal laser scanning microscopy of Tregs cocultured with 1.5 × 106 PMPs for 16 hours in the presence of anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2 and stained for CD41 (FITC, green) and CD25 (APC, red) or (G) P-selectin (FITC, green) and PSGL-1 (APC, red). Scale bar, 5 µm. Representative images are shown. Images were acquired with a TCS SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with an HCX-PL-APO 63× 1.2 water-immersion lens while cells were maintained at 37°C in HBS buffer. Leica LAS AF acquisition software was used.
PMP inhibition of Treg differentiation is P-selectin dependent. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) Intracellular IL-17 expression in Tregs that were cocultured with PMPs as a standard mixed culture (control) or separated by a porous membrane (transwell). (B) The presence of the platelet-specific marker CD41 was assessed on the CD4+ Tregs after coculture with increasing PMP concentrations. Each donor’s Tregs are shown as a dot representing the mean response of those Tregs to the 3 different donors’ PMPs. The mean is presented as bars. *P < .05. (C) Representative contour plots of PMPs stained with anti–CD41-PE and anti–P-selectin-FITC or isotype mAbs. The contour plots are representative of 3 donors. (D) Treg expression of PSGL-1 and CD25 at day 0 of culture. (E) Surface CD41, CD27, and intracellular expression of IL-17 on Treg cocultured with untreated, isotype, or anti–P-selectin mAb-neutralized PMPs. The graphs are representative of 2 Treg donors. Mean and SD are shown. *P < .05. (F) Confocal laser scanning microscopy of Tregs cocultured with 1.5 × 106 PMPs for 16 hours in the presence of anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2 and stained for CD41 (FITC, green) and CD25 (APC, red) or (G) P-selectin (FITC, green) and PSGL-1 (APC, red). Scale bar, 5 µm. Representative images are shown. Images were acquired with a TCS SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) equipped with an HCX-PL-APO 63× 1.2 water-immersion lens while cells were maintained at 37°C in HBS buffer. Leica LAS AF acquisition software was used.
As we observed clear staining for the platelet-specific marker CD41 on Tregs cocultured with PMPs (Figure 2B), even after repeated washing steps, we proposed that PMPs firmly bind to these cells and postulated that adhesion molecules, several of which are present on the PMP membrane,3 might be involved in this PMP–Treg contact-dependent interaction. Upon activation, platelets typically expose the adhesion molecule P-selectin, which they can pass onto the PMPs they generate.29 Like their parental cells, PMPs are able to bind immune cells expressing the P-selectin ligand P-selectin glycoprotein ligand-1 (PSGL-1).30 This led us to investigate whether P-selectin is involved in the observed PMP adhesion to Tregs and/or the inhibition of their differentiation. First, we established that the majority of PMPs (86.3% ± 2.4%) indeed expressed P-selectin (Figure 2C) and that Tregs expressed P-selectin ligand PSGL-1 at day 0 (Figure 2D). Subsequently, we performed neutralization experiments. Prior to coculture with Tregs, PMPs were incubated with an FITC-conjugated antagonistic anti–P-selectin mAb, an isotype control mAb, or in buffer only and subsequently washed. Although isotype mAb did not bind to the PMPs (0.4 ± 0.1%), PMPs were effectively bound by the anti–P-selectin mAb (80.6% ± 4.4%). The anti–P-selectin mAb was still present on the PMPs at day 7 of culture with Treg (77.4% ± 7.1%). Notably, the neutralization of P-selectin not only abrogated (CD41+) PMP adhesion to the Treg (Figure 2E) but also greatly blunted their inhibitory effect on Treg differentiation into IL-17–producing cells and loss of the Treg marker CD27 (Figure 2E). Neutralization of P-selectin also restored the loss of FOXP3 and CD62L and the increase in IFN-γ expression by Tregs during culture (supplemental Figure 9).
Confocal laser scanning microscopy of Tregs cocultured with PMPs confirmed the binding of CD41+ PMPs to the cell membrane of part of the Treg (Figure 2F). The majority of these PMPs had a diameter of 0.5 µm or less, whereas some were larger PMPs and/or PMP aggregates. In addition, adherent P-selectin+ PMPs colocalized with PSGL-1 on the Treg membrane (Figure 2G).
Then, we focused attention on platelet-associated immune signaling molecules. Because CD40-CD40L costimulation has been implicated in PMP-mediated signaling,23 we investigated the involvement of this pathway. However, neutralization of CD40 in culture with an antagonistic anti-CD40 mAb did not reduce the inhibitory effect of PMPs on Tregs (supplemental Figure 10).
Platelet factor 4 (PF4/CXCL4), predominantly expressed by platelets, is a CXCR3 ligand and triggers CXCR3-mediated signaling in activated T cells31 leading to inhibition of proliferation and IFN-γ secretion.32 Moreover, PF4 was found to be a negative regulator of TH17 differentiation.33 We therefore assessed CXCR3 expression on Tregs during culture and performed CXCR3 neutralization experiments. CXCR3 expression increased upon Treg activation (Figure 3A-C), and CXCR3 neutralization using an antagonistic CXCR3 mAb reduced the inhibitory effect of PMPs on the generation of IL-17–producing cells (Figure 3D). These data suggest that CXCR3 signaling contributes to the observed effects of PMPs on Treg differentiation.
CXCR3 neutralization leads to partial loss of PMP-mediated inhibition of IL-17 production by Tregs. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of 1.5 × 106 allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) Example dot plots of day 7 CXCR3 expression on Tregs. (B) CXCR3 expression on Tregs throughout culture. (C) CXCR3+ Tregs present in the CD41− and CD41+ subpopulations in time. (D) Day 7 intracellular staining of IL-17 on Tregs cultured with or without PMPs in the absence or presence of isotype or antagonistic anti-CXCR3 mAb. The percent PMP-mediated inhibition of IL-17–expressing Tregs is displayed in the graph. Results for 3 Treg donors are shown. Mean and SD are shown. *P < .05.
CXCR3 neutralization leads to partial loss of PMP-mediated inhibition of IL-17 production by Tregs. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads and recombinant human IL-2, IL-15, and IL-1β in the presence or absence of 1.5 × 106 allogeneic PMPs isolated from platelet apheresis units of 3 healthy donors. Flow cytometry analyses of intracellular cytokines were performed after stimulation with PMA and ionomycin, in the presence of Brefeldin A. (A) Example dot plots of day 7 CXCR3 expression on Tregs. (B) CXCR3 expression on Tregs throughout culture. (C) CXCR3+ Tregs present in the CD41− and CD41+ subpopulations in time. (D) Day 7 intracellular staining of IL-17 on Tregs cultured with or without PMPs in the absence or presence of isotype or antagonistic anti-CXCR3 mAb. The percent PMP-mediated inhibition of IL-17–expressing Tregs is displayed in the graph. Results for 3 Treg donors are shown. Mean and SD are shown. *P < .05.
PMPs selectively target CCR6+HLA-DR+ memory-like and IL-17–producing Tregs
Only a subset of peripheral blood–derived Tregs produces IL-17 upon stimulation.9-13 Insight into the features of this subset might guide us in the characterization and regulation of these cells. Sparked by the observation that the PMPs only associated with a subset of the Tregs (Figure 2B), while inhibiting IL-17 production, we argued that we might use this characteristic to pinpoint the IL-17 precursors within the whole Treg population.
To establish the identity of the Treg subset(s) targeted by the PMPs, and to determine whether this interaction occurs before Treg differentiation progresses, we followed PMP binding to Treg in time during a 7-day culture (Figure 4). Binding of PMPs to Tregs already occurred within 1 day of culture (Figure 4A). Concomitantly, an increase in the expression of the P-selectin ligand PSGL-1 was observed (Figure 4A).
PMPs selectively target CCR6+HLA-DR+ Tregs prior to and during activation. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads, recombinant human IL-2, IL-15, IL-1β, and 1.5 × 106 allogeneic PMPs isolated from platelet apheresis units of three healthy donors. Tregs were stained for surface markers and analyzed by flow cytometry at days 0, 1, 4, and 7. (A) CD41+ PMP-bound Tregs (n = 5 Treg donors) and the expression level of PSGL-1 on the Tregs (n = 3 PMP donors). (B) CCR6+HLA-DR+ Tregs present in the CD41− and CD41+ subpopulations. (C) Example CCR6/HLA-DR dot plots of PMP-negative (CD41−) and PMP-positive (CD41+) Treg. Mean and SD for 3 PMP donors are shown. *P < .05. Results representative of 5 Treg donors are shown. (D) Day 7 cell proliferation of Pacific Blue succinimidyl ester–labeled Tregs cocultured with and without PMPs was assessed. The division (Div.) index, proliferation (Prol.) index, and percentage of divided cells were determined for total Tregs and CCR6+HLA-DR+ Tregs using FlowJo 7.6. Results are shown for Tregs cultured alone and Tregs cocultured with PMPs of 3 different PMP donors.
PMPs selectively target CCR6+HLA-DR+ Tregs prior to and during activation. Tregs were isolated from healthy donors and cultured for 7 days with anti-CD3/anti-CD28 mAb–coated beads, recombinant human IL-2, IL-15, IL-1β, and 1.5 × 106 allogeneic PMPs isolated from platelet apheresis units of three healthy donors. Tregs were stained for surface markers and analyzed by flow cytometry at days 0, 1, 4, and 7. (A) CD41+ PMP-bound Tregs (n = 5 Treg donors) and the expression level of PSGL-1 on the Tregs (n = 3 PMP donors). (B) CCR6+HLA-DR+ Tregs present in the CD41− and CD41+ subpopulations. (C) Example CCR6/HLA-DR dot plots of PMP-negative (CD41−) and PMP-positive (CD41+) Treg. Mean and SD for 3 PMP donors are shown. *P < .05. Results representative of 5 Treg donors are shown. (D) Day 7 cell proliferation of Pacific Blue succinimidyl ester–labeled Tregs cocultured with and without PMPs was assessed. The division (Div.) index, proliferation (Prol.) index, and percentage of divided cells were determined for total Tregs and CCR6+HLA-DR+ Tregs using FlowJo 7.6. Results are shown for Tregs cultured alone and Tregs cocultured with PMPs of 3 different PMP donors.
CCR6-positive Tregs constitute a memory-like phenotype28 that was found to harbor the majority of Tregs with potential IL-17–producing capacity.9,10,20 Therefore, we assessed CD41 and CCR6 expression on the Tregs over time, combined with HLA-DR, which defines an activated Treg subset.12,34 Strikingly, a strong binding preference of PMPs was observed for CCR6+HLA-DR+ double-positive, but not single-positive, Tregs throughout days 1 to 7 of culture (Figure 4B-C). IL-17–producing cells were positive for both CCR6 and HLA-DR and expressed the TH17-associated transcription factor RORγT (supplemental Figure 11). Further phenotyping of the CD41+ Treg revealed that the majority expressed the functional Treg marker CD3935 and contained a higher proportion of CD161+ cells (data not shown), associated with potential IL-17–producing capacity.36 Coculture of Tregs with PMPs reduced Treg proliferation, particularly in CCR6+HLA-DR+ Tregs (Figure 4D). This provides a potential mechanism by which CCR6+HLA-DR+ Treg differentiation into IL-17–producing cells is inhibited. Finally, sorted CCR6+ and CCR6− Treg populations were cultured with and without PMPs (supplemental Figure 12). As expected, IL-17– and IFN-γ–producing Tregs were predominantly found in the CCR6+ Treg population (supplemental Figure 12B). Coculture of CCR6+ Treg with PMPs effectively inhibited the generation of IL-17– and IFN-γ–producing Tregs (supplemental Figure 12B), prevented the loss of FOXP3, and secured the suppressive capacity (supplemental Figure 12C-D).
Evidence for CD41+P-selectin+ Tregs in the circulation
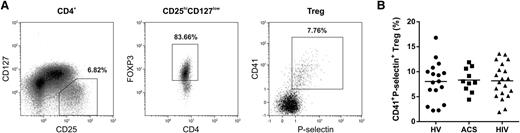
To provide evidence for human in vivo relevance of Treg/PMP interaction, we investigated the presence of PMP-bound Tregs in the circulation. We established that ∼8% of the peripheral blood Tregs from healthy individuals (n = 17) were CD41 and P-selectin double positive (Figure 5). Subsequently, we examined PMP–Treg binding in patients known to exhibit enhanced platelet activation and PMP formation in the circulation,29,37-39 ie, HIV-infected adults on stable combination antiretroviral therapy (n = 20) and patients with ACS (n = 10). We confirmed higher numbers of (PS+CD41+P-selectin+) PMPs in plasma of ACS patients (1.17 × 107 ± 0.93 × 107/mL) and HIV-infected individuals (6.20 × 107 ± 3.16 × 107/mL) compared with healthy volunteers (0.35 × 107 ± 0.36 × 107/mL). Notwithstanding the higher patient PMP levels, we found similar percentages of PMP-bound Tregs in peripheral blood compared with healthy individuals (Figure 5B). However, we did observe significantly less binding of HIV patient plasma PMPs to healthy control Tregs (Figure 6), whereas binding of PMPs from healthy individuals to Tregs of HIV-infected adults was comparable to their binding to Tregs of healthy volunteers (supplemental Figure 13). This suggests altered PMP binding capacity in these patients.
Detection of CD41+P-selectin+ Tregs in peripheral blood of healthy volunteers, HIV-infected adults, and patients with ACS. Flow cytometry analyses of peripheral blood obtained from patients with ACS (n = 10), HIV-infected adults on stable combination antiretroviral therapy (n = 20), and healthy volunteers (HV; n = 17) for surface CD4, CD127, CD25, CD41, P-selectin, and intracellular FOXP3 expression. (A) Representative example of CD4+CD25highCD127low/−FOXP3+ Treg gating and their exposure of CD41 and P-selectin in peripheral blood of a healthy volunteer. (B) Percentage of CD41 and P-selectin double-positive Tregs in the peripheral blood of ACS patients, HIV-infected adults, and healthy volunteers.
Detection of CD41+P-selectin+ Tregs in peripheral blood of healthy volunteers, HIV-infected adults, and patients with ACS. Flow cytometry analyses of peripheral blood obtained from patients with ACS (n = 10), HIV-infected adults on stable combination antiretroviral therapy (n = 20), and healthy volunteers (HV; n = 17) for surface CD4, CD127, CD25, CD41, P-selectin, and intracellular FOXP3 expression. (A) Representative example of CD4+CD25highCD127low/−FOXP3+ Treg gating and their exposure of CD41 and P-selectin in peripheral blood of a healthy volunteer. (B) Percentage of CD41 and P-selectin double-positive Tregs in the peripheral blood of ACS patients, HIV-infected adults, and healthy volunteers.
Reduced binding of plasma-derived PMPs from HIV-infected adults to Tregs. Tregs isolated from 5 healthy donors were cultured for 16 hours with recombinant human IL-2 in the presence or absence of total microparticle (MP) fractions isolated by differential centrifugation from platelet-poor plasma of patients with ACS (n = 3), HIV-infected adults on stable combination antiretroviral therapy (n = 3), and healthy volunteers (HV; n = 3). Tregs were stained for surface CD4, CD25, CD41, P-selectin, and intracellular FOXP3 and analyzed by flow cytometry. (A) Dot plots show the percentage of CD41 and P-selectin double-positive CD4+CD25highFOXP3+ Tregs. The dot plots were generated using merged data of 3 plasma microparticle donors. (B) The percentage of CD41 and P-selectin double-positive Tregs (n = 3 plasma microparticle donors). Representative results are shown. *P < .05.
Reduced binding of plasma-derived PMPs from HIV-infected adults to Tregs. Tregs isolated from 5 healthy donors were cultured for 16 hours with recombinant human IL-2 in the presence or absence of total microparticle (MP) fractions isolated by differential centrifugation from platelet-poor plasma of patients with ACS (n = 3), HIV-infected adults on stable combination antiretroviral therapy (n = 3), and healthy volunteers (HV; n = 3). Tregs were stained for surface CD4, CD25, CD41, P-selectin, and intracellular FOXP3 and analyzed by flow cytometry. (A) Dot plots show the percentage of CD41 and P-selectin double-positive CD4+CD25highFOXP3+ Tregs. The dot plots were generated using merged data of 3 plasma microparticle donors. (B) The percentage of CD41 and P-selectin double-positive Tregs (n = 3 plasma microparticle donors). Representative results are shown. *P < .05.
Discussion
Tregs constitute an essential component in the maintenance of tissue homeostasis and the prevention of (auto)inflammatory processes. Dysregulation of Treg function is associated with a myriad of pathologies (eg, psoriasis, multiple sclerosis, rheumatoid arthritis, and graft-versus-host disease).7,8,40 We and others have shown that Tregs may produce IFN-γ and IL-17 when stimulated under proinflammatory conditions9-13 and that these differentiated cells are found at sites of inflammation.19,41
In our search for immunomodulatory components that influence the Treg differentiation process, we hypothesized that PMPs might constitute a relevant force. Like their parental cells, PMPs are involved in hemostasis, maintenance of vascular health, and immunity.3,6,21,23,29,30,42
Here, we report that PMPs have the capacity to prevent the differentiation of Tregs into IL-17– and IFN-γ–producing cells, even in the presence of proinflammatory cytokines. The mechanism of action consists of rapid and selective P-selectin–dependent binding of PMPs to a specialized CCR6+HLA-DR+ memory-like Treg subset that is known to contain the majority of progenitor TH17-like cells,10,20 disrupting their proliferation and eventual differentiation into IL-17 producers. The few Tregs with bound PMPs that did acquire IL-17–producing capacity, were in a FOXP3+IL-17+ transitory state, likely due to the inhibitory effect of the PMPs. In addition, we found evidence for PMP-bound human Tregs in the circulation, suggesting a physiological function of this process.
Leukocytes play a central role in wound repair and are recruited after coagulation and the resulting vascular inflammation have been initiated.43 PSGL-1 is expressed on most leukocytes and mediates their recruitment to inflamed endothelium by binding P- and E-selectin on activated endothelial cells.44 P-selectin exposure by adherent activated platelets further assists leukocyte recruitment to the inflamed tissue.1 Moreover, activated platelets release P-selectin–positive PMPs,29 which also accumulate at sites of (vascular) injury,21 and are able to bind PSGL-1–positive leukocytes.30 We show that PMPs can bind peripheral PSGL-1–positive memory-like Tregs through P-selectin–mediated adhesion, possibly participating in their recruitment to sites of injury. Tregs were indeed found to be involved in local tissue repair by restricting the proinflammatory response in a lung injury mouse model.45 In patients with systemic vasculitis, vascular infiltration of TH17 and TH1 cells was enhanced, and fewer Tregs were observed in the lesions.46 Interestingly, FOXP3+ cells were shown to contribute to the IL-17 production in vascular lesions of these patients.41 Our observations raise the tantalizing possibility that PMPs might assist in the control and eventual resolution of inflammation during tissue repair by stabilizing the Treg phenotype and preventing their differentiation into TH1 or TH17-like cells in a proinflammatory microenvironment. One could argue that PMPs can easily enter inflamed tissue due to their small size and ability to interact with leukocytes, allowing them to hitchhike to the site of inflammation to exert their regulatory function. Increased PSGL-1 expression was observed on all Tregs, concomitantly with PMP binding upon Treg activation. Nevertheless, the PMPs showed a high preference for CCR6+HLA-DR+ double-positive Tregs, suggesting additional requirements for effective PMP binding. A possible explanation for this differential binding is that glycosyltransferases required to modify PSGL-1 to its active binding state are upregulated only upon T-cell activation.47
PSGL-1 engagement induces downstream signaling events that affect T-cell phenotype and function.44 Deletion of PSGL-1 increased T-cell proliferation and exacerbated inflammation in vivo.48 We also show that PMPs affect Treg proliferation, in particular within the CCR6+HLA-DR+ subpopulation that was preferentially targeted by PMPs. Thus, cell proliferation may be one of the mechanisms by which PMPs prevent IL-17 production by Tregs, which requires sufficient proliferation.9 Previously, activated washed platelets were shown to attenuate T-cell proliferation49,50 ; in our coculture system, we show that TRAP-activated washed platelets can inhibit proinflammatory cytokine production by Tregs. This might be due, at least in part, to PMP formation during coculture. Other scenarios that might explain the inhibitory effect include the involvement of additional signaling pathways next to PSGL-1. Our finding that CXCR3 signaling may be involved to a certain extent validates this line of thinking and deserves further attention, particularly because PF4, primarily expressed by platelets, triggers CXCR3-mediated signaling in activated T cells,31 leading to inhibition of proliferation and IFN-γ secretion,32 and was found to be a negative regulator of TH17 differentiation.33 Other possibilities include the transfer of genetic information from PMPs to Tregs5 or the initiation of Treg–Treg signaling by PMPs binding to multiple Tregs.30 The involvement of other adhesion molecules should also be considered, as exemplified by the ability of CD18 to keep Treg differentiation into IL-17–producing cells in check.51
Most PMPs in the circulation do not originate from platelets but are produced by megakaryocytes in the bone marrow.52,53 These megakaryocyte-derived microparticles are abundant in healthy individuals,3 whereas PMPs, identified by the expression of platelet activation markers P-selectin and/or CD63, are only observed during disease.29,42 This implies that although megakaryocyte-derived microparticles have a more systemic function, PMPs may act locally at sites of platelet activation and that each has its own distinct role in homeostasis.
Apparently, different platelet activation stimuli can yield regulatory PMPs, as we observed that PMPs formed by TRAP-activated washed platelets were as able as stored platelet concentrate–derived PMPs to curb Treg differentiation. We started out by using platelet apheresis units as the main source for our PMPs, because they are known to generate relatively large quantities of PMPs in a controlled and sterile environment.24 The majority of the isolated PMPs was P-selectin positive and thus originated from activated platelets in the concentrates. Therefore, our findings also add to the understanding of PMPs in platelet transfusion medicine, of which little is known thus far.54
Platelets55 and their microparticles6 have been reported to exert a proinflammatory function in the autoimmune diseases lupus and rheumatoid arthritis, respectively. However, a recent study shows that platelets can exert either a pro- or anti-inflammatory effect, depending on the type of inflammatory response they encounter.2 This observation, together with the alternative origin of the PMPs and a distinct cellular target (Tregs), could explain why we observe an anti-inflammatory effect instead. Of interest is our finding that binding of plasma-derived PMPs from HIV-infected individuals to Tregs was diminished in vitro. This suggests altered PMP binding, which although not directly obvious in peripheral blood, may be of relevance in the local tissue environment. Of note, increased IL-17 production by classical and nonclassical T cells is an increasingly recognized feature in HIV patients.56,57
In conclusion, we have identified a novel function of PMPs to selectively bind a specialized subset of TH17-like progenitor cells in the Treg compartment and inhibit their differentiation into potentially pathogenic effector cells. The direct involvement of the cell adhesion molecule P-selectin and its ligand PSGL-1 in this process suggests a role for PMPs in wound healing by participating in the regulation of the required inflammatory response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gaby Derksen and Rob Woestenenk (flow cytometry cell sorting) as well as Henk Tijssen (FOXP3 gene methylation analyses) from the Department of Laboratory Medicine, Radboud University Medical Center, Nijmegen.
Authorship
Contribution: S.D., H.J.P.M.K., G.J.C.G.M.B., and I.J. designed the research; S.D., B.v.C., W.A.v.d.H., X.H., and R.W. performed the experiments and together with H.J.P.M.K. and I.J. analyzed the data; S.D., H.J.P.M.K., G.J.C.G.M.B., and I.J. wrote the manuscript; and A.J.v.d.V. and I.E.D. provided access to reagents and patient material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irma Joosten, Laboratory of Medical Immunology (469), Radboud University Medical Center, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: irma.joosten@radboudumc.nl.
References
Author notes
B.v.C. and W.A.v.d.H. contributed equally to this study.