Key Points
Previous studies suggest that immune-mediated platelet clearance following transfusion represents an antibody-mediated process.
The results of this study demonstrate that CD8+ T cells can mediate platelet clearance independent of anti-platelet alloantibodies.
Abstract
Platelet transfusion provides an important therapeutic intervention in the treatment and prevention of bleeding. However, some patients rapidly clear transfused platelets, preventing the desired therapeutic outcome. Although platelet clearance can occur through a variety of mechanisms, immune-mediated platelet removal often plays a significant role. Numerous studies demonstrate that anti-platelet alloantibodies can induce significant platelet clearance following transfusion. In fact, for nearly 50 years, anti-platelet alloantibodies were considered to be the sole mediator of immune-mediated platelet clearance in platelet-refractory individuals. Although nonimmune mechanisms of platelet clearance can often explain platelet removal in the absence of anti-platelet alloantibodies, many patients experience platelet clearance following transfusion in the absence of a clear mechanism. These results suggest that other processes of antibody-independent platelet clearance may occur. Our studies demonstrate that CD8+ T cells possess the unique ability to induce platelet clearance in the complete absence of anti-platelet alloantibodies. These results suggest a previously unrecognized form of immune-mediated platelet clearance with significant implications in the appropriate management of platelet-refractory individuals.
Introduction
Although over 1.5 million platelet transfusions occur each year,1 a significant portion of individuals who receive platelets fail to achieve the desired therapeutic benefit due to accelerated platelet clearance.2,3 While clearance can occur through nonimmune-related mechanisms,4 many studies demonstrate the importance of immune-mediated clearance.2,3,5-8 Historically, immune-mediated platelet clearance, termed refractoriness, was attributed solely to anti-platelet alloantibodies predominately targeted to major histocompatibility complex (MHC) antigens.5,7 In the absence of detectable anti-platelet alloantibodies, platelet clearance is invariably considered nonimmune in nature.5,6 However, although studies demonstrate that some individuals can fail platelet therapy in the complete absence of detectable anti-platelet alloantibodies,2,3 nonimmune mechanisms often fail to fully explain platelet clearance, suggesting that immune-mediated platelet clearance may occur independent of anti-platelet alloantibodies.
Study design
Generating a mouse model for immune-mediated platelet clearance
C57BL/6 (H-2b) mice were immunized for 3 consecutive weeks by intraperitoneal injections of ∼10 × 106 total splenocytes from FVB (H-2q) mice. Generation of anti-platelet alloantibodies was confirmed by flow cross-match with FVB (H-2q) and C57BL/6 (H-2b) platelets. Immunized mice were transfused, as indicated, with platelets isolated as previously described9 from H2Kb-eGFP (B6GFP) (GFP+, H-2b) or FVB × H2Kb-eGFP (FVBGFP) (GFP+, H-2b, H-2q) mice. Subsequent green fluorescent protein–positive (GFP+) platelet clearance was assessed by flow cytometry at the times indicated following transfusion.
Assessing antibody-independent platelet refractoriness
To evaluate antibody-independent platelet clearance, μMT mice (B-cell–deficient C57BL/6, H-2b) were immunized and transfused with B6GFP or FVBGFP platelets, followed by evaluation of platelet clearance, as outlined in the previous paragraph. Absence of antibody was confirmed by western blot analysis of serum from naive and immunized C57BL/6 and μMT mice. Specific immune cell subsets were eliminated from immunized μMT mice prior to platelet transfusion by injection of monoclonal CD8-depleting antibody (clone 2.43) or NK1.1 monoclonal antibody (clone PK-136), respectively. Depletions were confirmed by flow cytometry.
Please refer to supplemental Materials (available on the Blood Web site) for detailed methodology.
Results and discussion
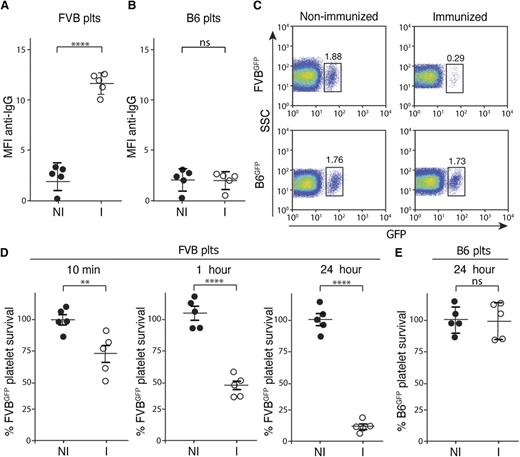
Although previous studies provide insight into the development of anti-platelet alloantibodies,2,9-14 few models exist to evaluate mechanisms of platelet refractoriness in transfused recipients. Therefore, we first developed a model to evaluate mechanisms whereby platelet clearance may occur following MHC alloimmunization. To accomplish this, C57BL/6 (H-2b) recipients were immunized with FVB (H-2q) splenocytes, which resulted in reproducible MHC alloimmunization monitored by evaluating anti-MHC alloantibody formation. Consistent with previous results, specific anti-H-2q alloantibodies were produced that recognized platelets isolated from FVB donors (Figure 1A). Importantly, these interactions appeared to be specific to FVB platelets, as serum from FVB-immunized C57BL/6 recipients failed to cross-react with platelets isolated from MHC-identical C57BL/6 donors (Figure 1B).
MHC-immunized recipients rapidly clear MHC-mismatched platelets. (A-B) Serum from nonimmunized C57BL/6 (H-2b) recipients (NI) or FVB (H-2q)-immunized C57BL/6 recipients (I) was incubated with FVB platelets (A) or C57BL/6 (B6) platelets (B) followed by detection of bound antibody by incubation with anti–immunoglobulin G (IgG) and flow cytometric examination (n = 5). (C) Nonimmunized or FVB-immunized C57BL/6 recipients were transfused with C57BL/6.GFP × FVB (FVBGFP) or C57BL/6.GFP (B6GFP) platelets followed by flow cytometric examination 24 hours later (gate = percentage of total platelets). (D-E) Percentage of FVBGFP (D) or B6GFP (E) platelets remaining, normalized to nonimmunized recipients, as indicated at various time points posttransfusion into nonimmunized (NI) or FVB-immunized (I) C57BL/6 recipients (n = 5). Significance was determined in panels A, B, D, and E by Student t test (**P ≤ .01, ****P ≤ .0001). MFI, mean fluorescence intensity; ns, no significance; plts, platelets; SSC, side scatter.
MHC-immunized recipients rapidly clear MHC-mismatched platelets. (A-B) Serum from nonimmunized C57BL/6 (H-2b) recipients (NI) or FVB (H-2q)-immunized C57BL/6 recipients (I) was incubated with FVB platelets (A) or C57BL/6 (B6) platelets (B) followed by detection of bound antibody by incubation with anti–immunoglobulin G (IgG) and flow cytometric examination (n = 5). (C) Nonimmunized or FVB-immunized C57BL/6 recipients were transfused with C57BL/6.GFP × FVB (FVBGFP) or C57BL/6.GFP (B6GFP) platelets followed by flow cytometric examination 24 hours later (gate = percentage of total platelets). (D-E) Percentage of FVBGFP (D) or B6GFP (E) platelets remaining, normalized to nonimmunized recipients, as indicated at various time points posttransfusion into nonimmunized (NI) or FVB-immunized (I) C57BL/6 recipients (n = 5). Significance was determined in panels A, B, D, and E by Student t test (**P ≤ .01, ****P ≤ .0001). MFI, mean fluorescence intensity; ns, no significance; plts, platelets; SSC, side scatter.
To avoid labeling strategies that may alter platelet clearance in an immune-independent fashion,15-18 we crossed C57BL/6 transgenics expressing GFP under a H-2Kb promoter19 with FVB, to generate C57BL/6.GFP × FVB progeny (FVBGFP) that express GFP and H-2q antigens. To determine whether FVB immunization increased FVBGFP platelet clearance, FVB-immunized C57BL/6 recipients were transfused with FVBGFP platelets and evaluated for platelet clearance at various time points posttransfusion. Transfused platelets could be detected as GFP and CD41-positive events immediately following transfusion (Figure 1C; supplemental Figure 1). Following transfusion into FVB-immunized C57BL/6 recipients, FVBGFP platelets rapidly declined, within an hour, to <80% of the initial FVBGFP platelet count detected immediately following transfusion (Figure 1D), consistent with rapid antibody-mediated platelet clearance in the clinical setting.5,6 Rapid platelet clearance did not appear to reflect an intrinsic defect in the survival of FVBGFP platelets, as this clearance phase failed to occur following transfusion into nonimmunized C57BL/6 recipients (Figure 1D). To determine whether platelet clearance required an MHC mismatch, C57BL/6-immunized recipients were transfused with MHC-matched GFP+ platelets (B6GFP). Similar to the inability of sera from FVB-immunized C57BL/6 recipients to recognize C57BL/6 platelets (Figure 1B), transfusion of B6GFP platelets into FVB-immunized recipients failed to result in any detectable changes in platelet clearance (Figure 1E).
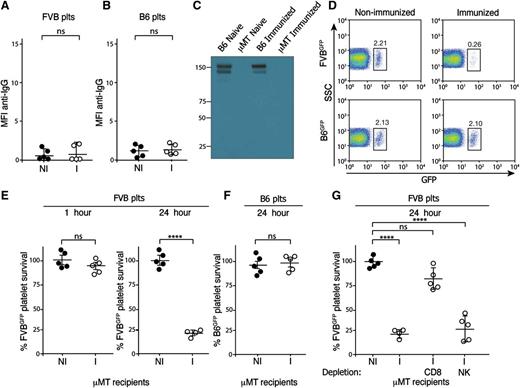
The correlation of anti-MHC alloantibody reactivity and platelet clearance corroborates decades of clinical observations that anti-platelet alloantibodies can mediate platelet clearance.5,7 However, as previous studies suggest that some individuals can experience accelerated platelet clearance in the absence of detectable anti-platelet alloantibodies,2,3,20 we next sought to examine potential mechanisms whereby antibody-independent, yet immune-mediated, platelet clearance might occur. As cellular rejection in the setting of transplantation can occur in the absence of anti-MHC alloantibodies,21 we next sought to determine whether a similar form of cellular immunity might mediate platelet clearance independent of anti-platelet alloantibodies. To examine this, we immunized μMT C57BL/6 (H-2b) recipients, which are deficient in B cells and therefore cannot generate antibodies,22 against FVB. Consistent with the lack of B cells in these mice, immunization failed to result in any detectable anti-FVB alloantibody (Figure 2A-B). Indeed, no antibodies could be detected in either immunized or nonimmunized recipients (Figure 2C). To determine whether FVB-immunized recipients possess the capacity to clear MHC-mismatched platelets, despite the lack of detectable anti-platelet alloantibodies, FVBGFP platelets were transfused into immunized or nonimmunized μMT C57BL/6 recipients. Although no detectable alterations in platelet clearance could be detected 1 hour following transfusion, significant clearance was observed 24 hours following transfusion into FVB-immunized recipients, whereas no alterations in clearance occurred in nonimmunized recipients (Figure 2D-E; supplemental Figure 2). Importantly, transfusion of MHC-matched B6GFP platelets into immunized or nonimmunized recipients failed to result in any detectable changes in platelet clearance (Figure 2D,F), strongly suggesting that the clearance of FVBGFP platelets reflected an immune-mediated process. These results suggest that although rapid platelet clearance may be antibody-mediated, significant immune-mediated platelet clearance can occur independent of anti-platelet alloantibodies.
CD8+ T-cell–mediated platelet clearance in immunized B-cell–deficient μMT recipients. (A-B) Serum from nonimmunized μMT (H-2b) recipients (NI) or FVB (H-2q)-immunized (I) μMT recipients was incubated with FVB platelets (A) or C57BL/6 (B6) platelets (B) followed by detection of bound antibody by incubation with anti-IgG and flow cytometric examination (n = 5). (C) Serum from nonimmunized or FVB-immunized μMT or C57BL/6 recipients was separated by gel electrophoresis under nonreducing conditions and analyzed by western blot analysis for immunoglobulin as indicated. (D) Nonimmunized or FVB-immunized μMT recipients were transfused with C57BL/6.GFP × FVB (FVBGFP) or C57BL/6.GFP (B6GFP) platelets followed by flow cytometric examination 24 hours later (n = 5) (gate = percentage of total platelets). (E-F) Percentage of FVBGFP (E) or B6GFP (F) platelets remaining, normalized to nonimmunized recipients as indicated at various time points posttransfusion into nonimmunized (NI) or FVB-immunized (I) μMT recipients (n = 5). (G) Percentage of FVBGFP platelets remaining, normalized to nonimmunized recipients, at 24 hours following transfusion, as indicated into nonimmunized (NI), FVB-immunized (I), CD8+ T-cell (CD8) depleted immunized or NK cell (NK) depleted immunized μMT recipients (n = 4-5). Significance was determined in panels A, B, E, and F by Student t test or by 1-way analysis of variance with the Tukey posttest in panel G (****P ≤ .0001; ns, no significance).
CD8+ T-cell–mediated platelet clearance in immunized B-cell–deficient μMT recipients. (A-B) Serum from nonimmunized μMT (H-2b) recipients (NI) or FVB (H-2q)-immunized (I) μMT recipients was incubated with FVB platelets (A) or C57BL/6 (B6) platelets (B) followed by detection of bound antibody by incubation with anti-IgG and flow cytometric examination (n = 5). (C) Serum from nonimmunized or FVB-immunized μMT or C57BL/6 recipients was separated by gel electrophoresis under nonreducing conditions and analyzed by western blot analysis for immunoglobulin as indicated. (D) Nonimmunized or FVB-immunized μMT recipients were transfused with C57BL/6.GFP × FVB (FVBGFP) or C57BL/6.GFP (B6GFP) platelets followed by flow cytometric examination 24 hours later (n = 5) (gate = percentage of total platelets). (E-F) Percentage of FVBGFP (E) or B6GFP (F) platelets remaining, normalized to nonimmunized recipients as indicated at various time points posttransfusion into nonimmunized (NI) or FVB-immunized (I) μMT recipients (n = 5). (G) Percentage of FVBGFP platelets remaining, normalized to nonimmunized recipients, at 24 hours following transfusion, as indicated into nonimmunized (NI), FVB-immunized (I), CD8+ T-cell (CD8) depleted immunized or NK cell (NK) depleted immunized μMT recipients (n = 4-5). Significance was determined in panels A, B, E, and F by Student t test or by 1-way analysis of variance with the Tukey posttest in panel G (****P ≤ .0001; ns, no significance).
Although a variety of cellular factors can mediate immunity independent of antibody function, CD8+ T cells represent the most classic and well recognized in the setting of transplantation.21 However, whether CD8+ T cells directly mediate platelet clearance following transfusion in MHC-alloimmunized individuals remains unknown. To examine this, FVB-immunized μMT recipients underwent CD8+ T-cell depletion prior to FVBGFP platelet transfusion (supplemental Figure 3). Although CD8+ T-cell depletion significantly attenuated clearance in immunized recipients (Figure 2G), injection of an isotype control failed to impact platelet removal (supplemental Figure 3). Similarly, although previous studies demonstrate that NK cells possess the ability to induce cellular removal, depletion of NK cells failed to significantly alter platelet clearance following transfusion into alloimmunized recipients (Figure 2G; supplemental Figures 3-4). These results suggest that CD8+ T cells can mediate platelet clearance independent of antibody effector function.
When patients fail to respond to platelet transfusion in the absence of detectable anti-platelet alloantibodies, nonimmune causes of platelet clearance become the primary diagnostic and therapeutic focus.23 However, our results indicate that immune-mediated platelet clearance can occur in the complete absence of detectable anti-platelet alloantibodies through a CD8+ T-cell–mediated process. The model system used in this study lacks B cells, allowing specific evaluation of CD8+ T-cell–mediated platelet clearance in the absence of anti-platelet alloantibodies. However, as patients typically possess intact B cells, future studies will be needed to evaluate whether CD8+ T cells mediate platelet clearance in platelet-refractory patients, especially those undergoing chemotherapy or displaying other conditions where platelet refractoriness is likely to occur. This is especially important when considering that diverse routes of HLA alloimmunization, such as pregnancy, transplantation, and transfusion can occur in patients,2,3,8,11-13,24 suggesting that various factors may influence the likelihood of CD8+ T-cell–mediated platelet clearance in any given patient. As previous results suggest that CD8+ T cells may be involved in the removal of platelets in patients with immune thrombocytopenia,20 the ability of CD8+ T cells to mediate platelet clearance in vivo may not only be limited to platelet transfusion, but may also contribute to impaired platelet levels in patients with thrombocytopenic conditions such as immune thrombocytopenia.
Although previous studies have evaluated the location and cells involved in antibody-mediated platelet clearance,25 it remains to be tested whether CD8+ T-cell–mediated platelet clearance likewise primarily relies on an intact spleen and requires common cell-mediated cytolytic molecules, such as perforin and granzyme. Regardless of the mechanisms whereby CD8+ T cells may clear transfused platelets, early studies suggest that MHC-matched platelet transfusion can result in better platelet counts even in the absence of detectable anti-platelet alloantibodies.26 These results, together with those of the present study, strongly suggest a role for CD8+ T cells in antibody-independent immune-mediated platelet clearance. Thus, this study provides important insight into a long-standing question surrounding platelet transfusion, with significant implications on mechanisms and treatment of non-antibody-mediated platelet removal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Burroughs Wellcome Trust Career Award for Medical Scientists and National Institutes of Health Common Fund Early Independence grant DP5OD019892 (S.R.S.).
Authorship
Contribution: S.R.S. and C.M.A. designed the research study and carried out and analyzed experiments together with S.R.P. and H.C.S.; A.M.W., C.A.T., and J.E.H. provided critical support; and C.M.A. and S.R.S. wrote the manuscript, which was additionally edited and commented on by the other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sean R. Stowell, Center for Transfusion Medicine and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, 105M Whitehead Building, 175 Michael St, Atlanta, GA 30322; e-mail: srstowe@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal