Key Points
Children with normalized TCD on transfusions were safely switched to hydroxyurea treatment, but trimestrial Doppler follow-up is required.
Allogeneic transplant allowed the safe stop of transfusions in all patients, even in those with abnormal velocities before transplant.
Abstract
Stroke risk in sickle cell anemia (SCA), predicted by high transcranial Doppler (TCD) velocities, is prevented by transfusions. We present the long-term follow-up of SCA children from the Créteil newborn cohort (1992-2012) detected at risk by TCD and placed on chronic transfusions. Patients with normalized velocities and no stenosis were treated with hydroxyurea, known to decrease anemia and hemolytic rate. Trimestrial Doppler was performed and transfusions restarted immediately in the case of reversion to abnormal velocities. Patients with a genoidentical donor underwent transplant. Abnormal time-averaged maximum mean velocities (TAMMV) ≥200 cm/s were detected in 92 SCA children at a mean age of 3.7 years (range, 1.3-8.3 years). No stroke occurred posttransfusion after a mean follow-up of 6.1 years. Normalization of velocities (TAMMV < 170 cm/s) was observed in 83.5% of patients. Stenosis, present in 27.5% of patients, was associated with the risk of non-normalization (P < .001). Switch from transfusions to hydroxyurea was prescribed for 45 patients, with a mean follow-up of 3.4 years. Reversion, predicted by baseline reticulocyte count ≥400 × 109/L (P < .001), occurred in 28.9% (13/45) patients at the mean age of 7.1 years (range, 4.3-9.5 years). Transplant, performed in 24 patients, allowed transfusions to be safely stopped in all patients and velocities to be normalized in 4 patients who still had abnormal velocities on transfusions. This long-term cohort study shows that transfusions can be stopped not only in transplanted patients but also in a subset of patients switched to hydroxyurea, provided trimestrial Doppler follow-up and immediate restart of transfusions in the case of reversion.
Introduction
Sickle cell anemia (SCA) is an inherited, severe disease with a high risk of cerebral vasculopathy responsible for strokes.1 In the early 1990s, transcranial Doppler (TCD) was introduced to detect patients at risk of stroke,2-7 a major progress in the management of SCA children in the last 20 years. Adams et al showed that patients with abnormally high cerebral velocities (time-averaged maximum mean velocity [TAMMV] ≥200 cm/s) have a 40% stroke risk within 3 years of detection,4 whereas the risk is lower than 2% in patients with normal velocities (<170 cm/s). Furthermore, the randomized Stroke Prevention Trial in Sickle Cell Anemia (STOP-1) study clearly demonstrated that long-term chronic transfusions allow significant reduction of the risk of stroke by 91% in patients with abnormal velocities.8 Detection of patients at risk of stroke by TCD led to the reduction of the incidence of first stroke in Californian SCA children from 0.88 per 100 person-years in 1991-1998 to 0.17 per 100 person-years in 2000.9 We reported in the Centre Hospitalier Intercommunal de Créteil (CHIC) newborn cohort study10 that screening with TCD at an early age and rapid initiation of transfusions in patients at risk reduced the risk of stroke by age 18 to 20 years from the previously reported rate of 11% to 12.8%1,11,12 to only 1.9%. However, we also showed that the cumulative risk of abnormal TCD was high, reaching a plateau of 30% by age 9 years,10 raising concerns about the high number of SCA children requiring chronic transfusions.
Considering the risk of severe side effects associated with chronic transfusions, such as iron overload13 and erythroid alloimmunization, the Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP-2) study (1998-2005) evaluated whether transfusions after at least 30 months could be safely suspended in patients with normalized velocities and no stenosis, and showed that stopping transfusions resulted in reversion to abnormal velocities and stroke occurrence.14 During the same period, because reports had shown that severe anemia is a risk factor for stroke1 and abnormal TCD3,15 and that hydroxyurea can improve hemoglobin levels16-18 and decrease velocities,18,19 the team at CHIC started in 199819 to treat with hydroxyurea SCA children with a history of abnormal TCD who had normalized cerebral velocities on chronic transfusion and had no stenosis. Transplant was recommended to patients with a genoidentical donor.20,21 We reported encouraging results in 2005, although the number of patients switched to hydroxyurea (n = 10) or transplanted (n = 6) was low,19 justifying longer follow-up.
We present here the follow-up in 92 cohort patients with a history of abnormal cerebral arterial velocities, as well as the cerebral outcome in 45 patients who were switched to hydroxyurea and 24 patients who underwent genoidentical hematopoietic stem cell transplant.
Patients and methods
The present study includes children from the CHIC newborn cohort with SCA (homozygous SCA, sickle cell/β0-thalassemia, or sickle cell/D-Punjab) born between May 1992 and December 2012. These children were screened by TCD imaging (TCDI)3,5-7 since the age of 12 to 18 months and had a history of abnormal TCDI defined by TAMMV ≥200 cm/s and at least 1 year of follow-up with TCDI. Hemoglobin levels were measured the same day as the TCDI assessment. Patients with acute anemia (acute splenic sequestration or erythroblastopenia with hemoglobin level <6 g/dL or 20% lower than the basal level) and abnormal velocities were transfused once and were assessed by TCDI 3 months later. If the TCDI control was normal, these patients were excluded from the study. All patients were clinically evaluated every 3 months and had a complete checkup every year.
TCDI via temporal window was systematically performed once a year since May 1992,10 and assessment via a submandibular window was added to the routine in June 2011.22,23 Patients with abnormal TCDI in the middle cerebral artery (MCA), anterior cerebral artery (ACA), internal carotid artery (ICA), or extracranial ICA (eICA) were placed on chronic transfusion and underwent cerebral magnetic resonance imaging (MRI)/magnetic resonance angiogram (MRA) after 2 transfusions. Cervical MRA was added in June 2011.22,23 Stenosis was defined by MRA as a ≥20% decrease in the lumen of arteries. Grading for stenosis (Table 1) was as described in the Stroke with Transfusions Changing to Hydroxyurea study.24 Silent infarcts were defined as ischemic lesions of at least 3 mm by MRI in patients without a history of cerebrovascular events.
Site of abnormal velocities and stenosis
| Abnormal TCDI site . | n . | Site of stenosis . | |||
|---|---|---|---|---|---|
| Intra . | Extra . | Intra + Extra . | Total . | ||
| Intracranial | |||||
| Isolated MCA | 66 | 12 | 3 | 0 | 15 |
| Isolated ICA | 8 | 2 | 0 | 0 | 2 |
| Isolated ACA | 3 | 0 | 0 | 0 | 0 |
| MCA + ACA | 3 | 1 | 0 | 0 | 1 |
| Extracranial | |||||
| Isolated eICA | 10 | 1 | 4 | 1 | 6 |
| Intra + Extra | |||||
| ACA + eICA | 1 | 0 | 0 | 1 | 1 |
| MCA + eICA | 1 | 0 | 1 | 0 | 1 |
| Total | 92 | 16 | 8 | 2 | 26 |
| Abnormal TCDI site . | n . | Site of stenosis . | |||
|---|---|---|---|---|---|
| Intra . | Extra . | Intra + Extra . | Total . | ||
| Intracranial | |||||
| Isolated MCA | 66 | 12 | 3 | 0 | 15 |
| Isolated ICA | 8 | 2 | 0 | 0 | 2 |
| Isolated ACA | 3 | 0 | 0 | 0 | 0 |
| MCA + ACA | 3 | 1 | 0 | 0 | 1 |
| Extracranial | |||||
| Isolated eICA | 10 | 1 | 4 | 1 | 6 |
| Intra + Extra | |||||
| ACA + eICA | 1 | 0 | 0 | 1 | 1 |
| MCA + eICA | 1 | 0 | 1 | 0 | 1 |
| Total | 92 | 16 | 8 | 2 | 26 |
Among the 18 patients with intracranial stenosis, 17 had abnormal TCDI in MCA or ICA (STOP criteria), whereas 1 patient had abnormal ACA velocities associated with abnormal eICA velocities and stenosis in ACA and eICA. Of note, 3 patients with isolated MCA abnormal velocities had also eICA stenosis, and 2 patients with isolated eICA abnormal velocities had intracranial stenosis (associated with extracranial in 1). Grading of stenosis detected 2 patients with grade 5, 2 with grade 4, 2 with grade 3, 3 with grade 2, and 17 with grade 1.
Extra, extracranial; intra, intracranial.
Chronic transfusions for patients with abnormal TCDI aimed at maintaining sickle hemoglobin level below 30% and hemoglobin between 9 and 11 g/dL. Patients with normalized velocities (<170 cm/s) and no stenosis were placed on hydroxyurea, given at a dose of 12 mg/kg per day during the first month and then 23 mg/kg per day, and subsequently increased until the maximal tolerated dose (MTD).25 Transfusions overlapped with the start of hydroxyurea for at least 2 months, TCDI was controlled every 3 months, and transfusions were immediately reinstated in the case of abnormal TCDI recurrence or new evidence of stenosis. Transplant was recommended to patients who had an available HLA-identical sibling, first to patients who still had abnormal TCDI despite transfusions, and then from 2003 on, to those who had abnormal TCDI reversion on hydroxyurea and those with normalized velocities. Parental written informed consent was obtained before transplant and before hydroxyurea therapy because it is a chemotherapeutic agent. Use of the database was approved for this project by the Créteil Institutional Review Board. According to the rules and regulations in effect in France, no institutional review board approval or oversight committee was needed for this standard of care in which hydroxyurea therapy was given to patients with normalized velocities and no stenosis, and that included trimestrial TCDI control and immediate transfusion re-initiation in the case of reversion to abnormal TCDI.
Statistical analysis
Participant characteristics at baseline were summarized through the use of percentages, mean ± standard deviation, or median, with 25th and 75th percentiles denoted Q1-Q3. Ninety-five percent confidence intervals (CIs) around point estimates were computed. Fisher’s exact tests were used to compare proportions, and Wilcoxon rank sum tests were used to compare continuous distributions. The date of the first and last TCDI assessment defined entry into the study and end point, respectively. For Kaplan-Meier (KM) estimates of velocity normalization, participants were censored at the date of normalized TCDI or at the date of the last TCDI in the absence of normalization. Curves for the time to normalization as a function of the presence or absence of stenosis were compared by the log-rank test. For KM estimates of reversion to abnormal TCDI on hydroxyurea therapy, participants were censored at the date of reversion or at the date of the last TCDI in absence of reversion. To assess predictive risk factors for absence of normalization of velocities or reversion to abnormal TCDI on hydroxyurea therapy, we used the Cox regression analysis with estimated hazard ratio (HR) and 95% CI. Univariate models were fitted, and all variables associated with the outcome at the 10% level were retained for introduction into a multivariate model. All statistical tests were 2 sided, with P ≤ .05 denoting statistical significance. Statistical analysis was performed with SPSS version 22 and MedCalc version 15.2 (Belgium) software packages.
Results
Patients with a history of abnormal cerebral velocities (n = 92)
A total of 309 SCA children (296 homozygous, 10 sickle cell/β0-thalassemia, 3 sickle cell/D-Punjab) followed since birth and with at least 1 year follow-up with TCDI were included in the present study, providing 2684 patient-years of TCDI follow-up. Mean age was 1.7 ± 0.6 years (range, 0.5-3.2 years) at first TCDI and 8.1 ± 4.5 years (range, 2-19 years) at last TCDI. Baseline biological parameters are shown in Tables 2 and 3.
Biological characteristics in patients with and without abnormal velocities defined as ≥200 cm/s
| . | Patients without abnormal velocities (N = 217) . | Patients with abnormal velocities . | ||||||
|---|---|---|---|---|---|---|---|---|
| Isolated intracranial (N = 79) . | Isolated extracranial (N = 10) . | |||||||
| n . | % . | n . | % . | P* . | n . | % . | P† . | |
| Gender | ||||||||
| F | 115 | 53.0 | 38 | 48.1 | NS | 2 | 20.0 | .053 |
| M | 102 | 47 | 41 | 51.9 | 8 | 80.0 | ||
| G6PD‡ | ||||||||
| Deficiency | 16 | 8.4 | 12 | 17.4 | .041 | 1 | 14.3 | |
| Normal | 174 | 91.6 | 57 | 82.6 | 6 | 85.7 | ||
| α Genes | ||||||||
| 2 | 25 | 12.6 | 4 | 5.5 | 1 | 11.1 | ||
| 3 | 73 | 36.7 | 17 | 23.3 | 3 | 33.3 | ||
| 4 | 100 | 50.3 | 52 | 71.2 | 5 | 55.6 | ||
| 5 | 1 | 0.5 | 0 | |||||
| α-Thalassemia | ||||||||
| Absent | 101 | 50.8 | 52 | 71.2 | .004 | 5 | 55.6 | NS |
| Present | 98 | 49.2 | 21 | 28.8 | 4 | 44.4 | ||
| β Haplotype | ||||||||
| CAR/CAR | 65 | 34.8 | 33 | 45.8 | NS | 4 | 44.4 | NS |
| BEN/BEN | 53 | 28.3 | 15 | 20.8 | 1 | 11.1 | ||
| SEN/SEN | 21 | 11.2 | 5 | 6.9 | 3 | 33.3 | ||
| Others | 48 | 25.7 | 19 | 26.4 | 1 | 11.1 | ||
| . | Patients without abnormal velocities (N = 217) . | Patients with abnormal velocities . | ||||||
|---|---|---|---|---|---|---|---|---|
| Isolated intracranial (N = 79) . | Isolated extracranial (N = 10) . | |||||||
| n . | % . | n . | % . | P* . | n . | % . | P† . | |
| Gender | ||||||||
| F | 115 | 53.0 | 38 | 48.1 | NS | 2 | 20.0 | .053 |
| M | 102 | 47 | 41 | 51.9 | 8 | 80.0 | ||
| G6PD‡ | ||||||||
| Deficiency | 16 | 8.4 | 12 | 17.4 | .041 | 1 | 14.3 | |
| Normal | 174 | 91.6 | 57 | 82.6 | 6 | 85.7 | ||
| α Genes | ||||||||
| 2 | 25 | 12.6 | 4 | 5.5 | 1 | 11.1 | ||
| 3 | 73 | 36.7 | 17 | 23.3 | 3 | 33.3 | ||
| 4 | 100 | 50.3 | 52 | 71.2 | 5 | 55.6 | ||
| 5 | 1 | 0.5 | 0 | |||||
| α-Thalassemia | ||||||||
| Absent | 101 | 50.8 | 52 | 71.2 | .004 | 5 | 55.6 | NS |
| Present | 98 | 49.2 | 21 | 28.8 | 4 | 44.4 | ||
| β Haplotype | ||||||||
| CAR/CAR | 65 | 34.8 | 33 | 45.8 | NS | 4 | 44.4 | NS |
| BEN/BEN | 53 | 28.3 | 15 | 20.8 | 1 | 11.1 | ||
| SEN/SEN | 21 | 11.2 | 5 | 6.9 | 3 | 33.3 | ||
| Others | 48 | 25.7 | 19 | 26.4 | 1 | 11.1 | ||
Three of 92 patients had both intra- and extracranial abnormal TCDI and are not reported in this table.
BEN, Benin; CAR, Central African Republic; G6PD, glucose-6-phosphate dehydrogenase; NS, not significant SEN, Senegal.
Comparison of variable distribution between patients without abnormal velocities and those with isolated intracranial abnormal velocities. Bold type denotes statistically significant.
Comparison of variable distribution between patients without abnormal velocities and those with isolated extracranial abnormal velocities.
G6PD activity was assessed at baseline, a minimum of 3 mo away from a transfusion by reduction of nicotinamide adenine dinucleotide phosphate (NADP) to NADPH, measured by UV spectrophotometry.
Patients with and without abnormal (≥200 cm/s) velocities: biological parameters at baseline
| Parameters at baseline . | Patients without abnormal velocities (N = 217) . | Patients with abnormal velocities . | ||||||
|---|---|---|---|---|---|---|---|---|
| Isolated intracranial (N = 79) . | Isolated extracranial (N = 10) . | |||||||
| n . | Median (Q1-Q3) . | n . | Median (Q1-Q3) . | P* . | n . | Median (Q1-Q3) . | P . | |
| White blood cell count, 109/L | 196 | 13.300 (10.300-16.100) | 72 | 15.400 (12.525-20.075) | <.001 | 9 | 13.400 (10.950-15.450) | NS |
| Neutrophil count, 109/L | 192 | 4.805 (3.287-7.052) | 67 | 5.620 (4.350-8.210) | .028 | 9 | 4.240 (3.260-4.240) | NS |
| Platelet count, 109/L | 194 | 355.000 (274.000-431.750) | 71 | 332.000 (263.000-410.000) | NS | 9 | 386.000 (278.000-435.000) | NS |
| Hemoglobin level, g/dL | 196 | 8.2 (7.5-9.2) | 72 | 7.5 (6.8-8.1) | <.001 | 9 | 8.0 (7.3-9.5) | NS |
| Hematocrit, % | 194 | 24.8 (22.3-28.0) | 69 | 22.4 (20.9-24.4) | <.001 | 9 | 22.8 (21.6-29.1) | NS |
| Mean corpuscular volume, fL | 194 | 76.2 (70.7-82.1) | 69 | 80.4 (75.3-85.2) | .002 | 9 | 76.0 (71.0-81.8) | NS |
| Reticulocyte count, 109/L | 189 | 273.700 (197.350-344.000) | 68 | 323.550 (261.000-417.225) | <.001 | 9 | 272.000 (207.850-319.600) | NS |
| Lactate dehydrogenase, IU/L† | 167 | 701 (551-1021) | 57 | 854 (561-1205) | NS | 8 | 766 (587-884) | NS |
| Bilirubin, μmol/L | 158 | 29 (21-39) | 54 | 35 (25-50) | NS | 6 | 28.5 (19.5-37.7) | NS |
| Fetal hemoglobin, % | 188 | 16.5 (9.5-21.6) | 64 | 13.4 (9.0-18.0) | .012 | 9 | 15.1 (10.8-21.6) | NS |
| Peripheral hemoglobin saturation, % | 87 | 98 (97-100) | 23 | 99 (96-100) | NS | 5 | 98.0 (95.5-99.5) | NS |
| Parameters at baseline . | Patients without abnormal velocities (N = 217) . | Patients with abnormal velocities . | ||||||
|---|---|---|---|---|---|---|---|---|
| Isolated intracranial (N = 79) . | Isolated extracranial (N = 10) . | |||||||
| n . | Median (Q1-Q3) . | n . | Median (Q1-Q3) . | P* . | n . | Median (Q1-Q3) . | P . | |
| White blood cell count, 109/L | 196 | 13.300 (10.300-16.100) | 72 | 15.400 (12.525-20.075) | <.001 | 9 | 13.400 (10.950-15.450) | NS |
| Neutrophil count, 109/L | 192 | 4.805 (3.287-7.052) | 67 | 5.620 (4.350-8.210) | .028 | 9 | 4.240 (3.260-4.240) | NS |
| Platelet count, 109/L | 194 | 355.000 (274.000-431.750) | 71 | 332.000 (263.000-410.000) | NS | 9 | 386.000 (278.000-435.000) | NS |
| Hemoglobin level, g/dL | 196 | 8.2 (7.5-9.2) | 72 | 7.5 (6.8-8.1) | <.001 | 9 | 8.0 (7.3-9.5) | NS |
| Hematocrit, % | 194 | 24.8 (22.3-28.0) | 69 | 22.4 (20.9-24.4) | <.001 | 9 | 22.8 (21.6-29.1) | NS |
| Mean corpuscular volume, fL | 194 | 76.2 (70.7-82.1) | 69 | 80.4 (75.3-85.2) | .002 | 9 | 76.0 (71.0-81.8) | NS |
| Reticulocyte count, 109/L | 189 | 273.700 (197.350-344.000) | 68 | 323.550 (261.000-417.225) | <.001 | 9 | 272.000 (207.850-319.600) | NS |
| Lactate dehydrogenase, IU/L† | 167 | 701 (551-1021) | 57 | 854 (561-1205) | NS | 8 | 766 (587-884) | NS |
| Bilirubin, μmol/L | 158 | 29 (21-39) | 54 | 35 (25-50) | NS | 6 | 28.5 (19.5-37.7) | NS |
| Fetal hemoglobin, % | 188 | 16.5 (9.5-21.6) | 64 | 13.4 (9.0-18.0) | .012 | 9 | 15.1 (10.8-21.6) | NS |
| Peripheral hemoglobin saturation, % | 87 | 98 (97-100) | 23 | 99 (96-100) | NS | 5 | 98.0 (95.5-99.5) | NS |
Average biological parameters were obtained at baseline after the age of 12 mo and before the age of 3 y, a minimum of 3 mo away from a transfusion, 1 mo from a painful episode and before any intensive therapy (hydroxyurea, transfusion program, or stem cell transplant).
Bold type denotes statistically significant.
Lactate dehydrogenase normal range for the institution is 135-225 IU/L.
Out of these 309 SCA children, 92 had a history of abnormal TCDI at the mean age of 3.7 ± 1.5 years (range, 1.3-8.3 years). Mean velocities were 210 ± 17 cm/s, with a 75th percentile ≥215 cm/s. Arteries affected were right (n = 42) and left (n = 29) MCA; right (n = 4) and left (n = 3) ACA; right (n = 2) and left (n = 7) ICA; and right (n = 9) and left (n = 4) eICA. Of note, 8 patients had several affected arteries. Seventy-eight patients had abnormal TCDI in MCA/ICA (STOP criteria), whereas isolated abnormal TCDI in ACA and eICA were observed in 4 and 10 patients, respectively.
The MRI/MRA, performed in 91 of 92 patients within 3 to 6 months after the first abnormal TCDI, was normal in 65 of 91 (71.4%) patients but showed presence of stenosis in 26 of 91 (28.6%) patients. Silent infarcts were present in 20 of 91 patients (Table 1). Velocities were significantly higher in those with stenosis (222 ± 31 vs 207 ± 12 cm/s; P = .002), and velocities ≥215 cm/s were significantly associated with stenosis (P = .034). Blood parameters at baseline were not significantly different between patients with abnormal velocities plus stenosis and those with no stenosis.
Outcome of patients with abnormal velocities
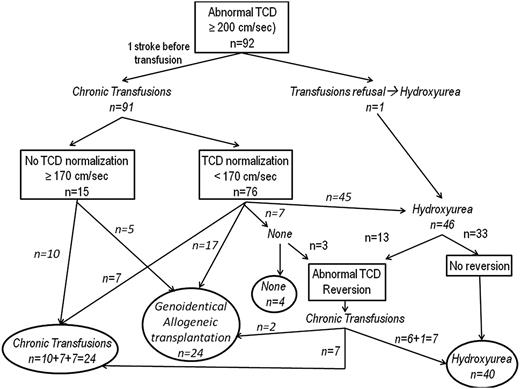
The mean age at the last TCDI for the 92 patients was 9.9 ± 3.7 years (range, 2.4-18.9 years). Between the first abnormal TCDI and the last TCDI assessment, the mean follow-up was 6.1 ± 3.6 years (range, 0.7-16.0 years) (Figure 1).
Flowchart of the study of patients from the newborn cohort with a history of abnormal velocities. Outcome of velocities after first normalization. Chronic transfusion was continued in 7 patients: 1 patient developed conditional velocities after initial normalization on transfusion, 2 patients with an identical donor are awaiting transplant, and 4 patients are scheduled for the switch to hydroxyurea. Chronic transfusion was stopped, and no new treatment introduced in 7 patients. Three of these had hypersplenism and were splenectomized, and it was decided to only observe the outcome. The parents of the 4 other patients refused the switch to hydroxyurea. After transfusion discontinuation, 3 of 7 patients (including 1 splenectomized) had reversion to abnormal TCDI and were placed again on transfusion, but 1 having a genoidentical cord blood donor was later transplanted. Transplant was performed in 17 patients who had a genoidentical donor. They were maintained on transfusion until transplant. All still have normal velocities posttransplant. Switch to hydroxyurea was prescribed in 45 patients (see the text).
Flowchart of the study of patients from the newborn cohort with a history of abnormal velocities. Outcome of velocities after first normalization. Chronic transfusion was continued in 7 patients: 1 patient developed conditional velocities after initial normalization on transfusion, 2 patients with an identical donor are awaiting transplant, and 4 patients are scheduled for the switch to hydroxyurea. Chronic transfusion was stopped, and no new treatment introduced in 7 patients. Three of these had hypersplenism and were splenectomized, and it was decided to only observe the outcome. The parents of the 4 other patients refused the switch to hydroxyurea. After transfusion discontinuation, 3 of 7 patients (including 1 splenectomized) had reversion to abnormal TCDI and were placed again on transfusion, but 1 having a genoidentical cord blood donor was later transplanted. Transplant was performed in 17 patients who had a genoidentical donor. They were maintained on transfusion until transplant. All still have normal velocities posttransplant. Switch to hydroxyurea was prescribed in 45 patients (see the text).
Stroke.
One patient, who was very young at the first abnormal TCDI (1.5 years), experienced an overt stroke 1 month post-TCDI, just before the TCDI control and the first transfusion.10 None of the other 91 patients had a stroke during the follow-up.
Outcome of velocities on chronic transfusion.
Except for 1 patient whose parents refused any transfusions and who was directly treated with hydroxyurea, the other 91 patients with abnormal TCDI were placed on chronic transfusion.
A return to normal velocities (<170 cm/s) was observed in 76 of 91 (83.5%) patients at the median age of 5.3 ± 1.9 years (range, 1.8-10.8 years) and after a mean duration of chronic transfusion of 1.4 ± 1.3 years (range, 0.3-6.9 years).
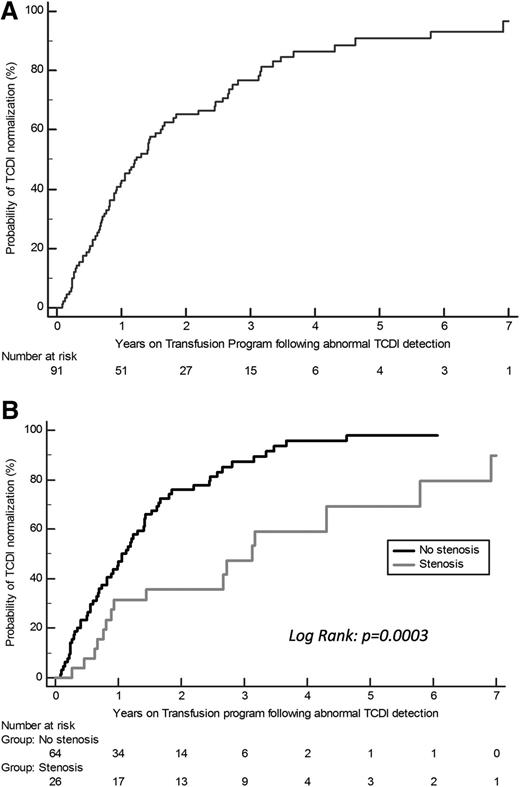
The KM estimate of the cumulative proportion of patients with TCDI normalization on transfusion is shown in Figure 2A. Despite chronic transfusion, velocities did not normalize in 15 patients. They were maintained on transfusion, except for 5 who had an available genoidentical donor and were transplanted.
Patients with abnormal velocities. (A) The KM estimate of the cumulative proportion of patients with abnormal TCDI who converted to normal TCDI while receiving transfusions was 43% (95% CI, 32.6-53.4) at 1 year, 66.5% (95% CI, 56.3-76.7) at 2 years, and 86.4% (95% CI, 78.2-94.6) at 4 years. (B) KM estimates of the probability of TCDI normalization on chronic transfusion depending on the presence of stenosis at first MRA after abnormal TCDI detection. Significant differences were observed depending on the presence of stenosis at the first MRA as after 2 years of transfusion: 25.3% (95% CI, 14.5-36.1) of patients without stenosis still had abnormal velocities vs 61.6% (95% CI, 41.4-81.8) of those with stenosis (log-rank, P < .001). A trend to a significant association with the stenosis grading was observed (P = .058); of note, no TCDI normalization was observed in the 4 patients with grading ≥4.
Patients with abnormal velocities. (A) The KM estimate of the cumulative proportion of patients with abnormal TCDI who converted to normal TCDI while receiving transfusions was 43% (95% CI, 32.6-53.4) at 1 year, 66.5% (95% CI, 56.3-76.7) at 2 years, and 86.4% (95% CI, 78.2-94.6) at 4 years. (B) KM estimates of the probability of TCDI normalization on chronic transfusion depending on the presence of stenosis at first MRA after abnormal TCDI detection. Significant differences were observed depending on the presence of stenosis at the first MRA as after 2 years of transfusion: 25.3% (95% CI, 14.5-36.1) of patients without stenosis still had abnormal velocities vs 61.6% (95% CI, 41.4-81.8) of those with stenosis (log-rank, P < .001). A trend to a significant association with the stenosis grading was observed (P = .058); of note, no TCDI normalization was observed in the 4 patients with grading ≥4.
Risk factors for absence of normalization on chronic transfusion.
The maximum TAMMV (224 ± 33 vs 209 ± 16 cm/s; P = .012) was significantly higher, and the presence of stenosis was significantly more frequent (10/15 vs 15/76; P = .001), in patients who did not normalize velocities, but the age at abnormal TCDI detection (3.5 ± 1.8 vs 3.8 ± 1.5 years) was not different (Figure 2B).
The probability of TCDI normalization was significantly lower in presence of stenosis (log-rank, P = .001) (Figure 2B).
Multivariate Cox regression analysis, with maximum velocities and stenosis as variables, retained only the presence of stenosis at first MRA (HR, 2.6; 95% CI, 1.5-4.7; P = .001) as predictive of the lack of normalization.
Outcome of velocities after first normalization.
Hydroxyurea was initiated in 45 patients after TCDI normalization on transfusions after a mean duration of chronic transfusion of 2.7 ± 1.7 years (range, 0.3-7.8 years) and at a mean age of 6.4 ± 1.9 years (range, 3.1-11.1 years). The mean dose of hydroxyurea was 24.6 ± 2.1 mg/kg per day (range, 21.2-30.1 mg/kg per day) (Figure 1).
The patient with no stenosis at first MRA who was directly placed on hydroxyurea continued to receive hydroxyurea, and velocities normalized on hydroxyurea. At the first MRA, 8 of 45 patients had stenosis that disappeared in 7 of the 8 patients after chronic transfusion, and 1 of 45 patients had inadequate MRA. Thus, at hydroxyurea initiation, 1 patient still had inadequate MRA and only 1 patient had stenosis. However, hydroxyurea was given to this patient upon return to Africa where chronic transfusion was not available.
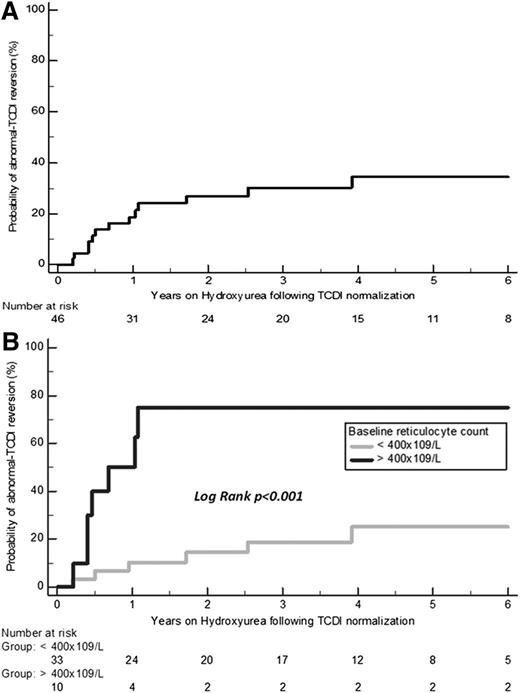
Abnormal TCDI reversion was observed in 13 of 46 (28.3%) patients on hydroxyurea at the mean age of 7.1 ± 1.6 years (range, 4.3-9.5 years) after a mean treatment duration of 1.1 ± 1.1 years (range, 0.2-3.9 years). Reversion occurred during the first 6 months on hydroxyurea in 4 of 13 patients. KM estimates of the probability of reversion on hydroxyurea are shown in Figure 3A. The mean dose of hydroxyurea at reversion was 23.1 ± 0.6 mg/kg per day, and 7 of the 13 patients had reached the MTD. One patient reverted to abnormal velocities after 3.9 years on hydroxyurea. Nonadherence was highly suspected because mean corpuscular volume and percentage fetal hemoglobin were lower at reversion time than previously (79.2 vs 84.9 fL and 16.4% vs 30%, respectively).
KM estimates of the probability of abnormal TCDI reversion on hydroxyurea. (A) KM estimates in the 46 patients. (B) KM estimates depending on the baseline reticulocyte count of <400 × 109/L or ≥400 × 109/L recorded before age 3 years. The KM-estimated cumulative risk of reversion in patients on hydroxyurea was 19.2% (95% CI, 7-31.4) at 1 year and 27.4% (95% CI, 13.2-41.6) at 2 years.
KM estimates of the probability of abnormal TCDI reversion on hydroxyurea. (A) KM estimates in the 46 patients. (B) KM estimates depending on the baseline reticulocyte count of <400 × 109/L or ≥400 × 109/L recorded before age 3 years. The KM-estimated cumulative risk of reversion in patients on hydroxyurea was 19.2% (95% CI, 7-31.4) at 1 year and 27.4% (95% CI, 13.2-41.6) at 2 years.
The 13 patients were immediately placed again on transfusion. Subsequently, 2 of 13 with a genoidentical donor were transplanted; 6 of 13 after new TCDI normalization were again treated with hydroxyurea. However, 2 of 6 patients had a second reversion, requiring initiation of a third round of chronic transfusion, but were again treated with hydroxyurea after the latest normalization.
Treatments administered to the other 47 patients are reported in the legend of Figure 1.
Risk factors for abnormal velocities reversion on hydroxyurea.
Occurrence of reversion on hydroxyurea was not significantly associated with the duration of chronic transfusion needed to obtain TCDI normalization or with age and maximum velocities at the time of abnormal TCDI.
Multivariate Cox regression analysis, including all blood parameters recorded at baseline before age 3 years as variables (Table 4) retained only baseline reticulocyte count as a significant predictive factor for reversion on hydroxyurea (HR, 1.005 per 1 × 109/L increase; 95% CI, 1.001-1.009; P = .010). Furthermore, baseline reticulocyte count ≥400 × 109/L was a highly significant predictive factor for reversion on hydroxyurea (HR, 6.3; 95% CI, 1.8-21.7; P = .001) (Figure 3B).
Comparison of blood parameters (recorded at baseline before age 3 and on hydroxyurea) of patients who reversed to abnormal TCDI on hydroxyurea vs those who did not
| Blood parameters . | Patients placed on hydroxyurea (N = 46) . | ||
|---|---|---|---|
| No reversion (n = 33), median (Q1-Q3) . | Reversion (n = 13), median (Q1-Q3) . | P* . | |
| At baseline | |||
| White blood cell count, 109/L | 14.800 (12.700-17.700) | 18.200 (11.975-20.950) | .051 |
| Neutrophil count, 109/L | 4.860 (4.205-7.297) | 6.225 (4.325-9.407) | .04 |
| Platelet count, 109/L | 354.000 (311.250-422.750) | 315.000 (262.250-349.750) | .189 |
| Hemoglobin level, g/dL | 7.8 (7.3-8.6) | 7.4 (6.7-8.0) | .074 |
| Hematocrit, % | 22.6 (21.5-25.7) | 22.0 (20.6-23.7) | .142 |
| Mean corpuscular volume, fL | 79.0 (74.4-83.1) | 82.7 (78.7-87.4) | .073 |
| Reticulocyte count, 109/L | 297.000 (248.200-355.000) | 448.100 (317.880-524.750) | .007 |
| Lactate dehydrogenase, IU/L | 974 (631-1249) | 998 (611-1267) | .656 |
| Bilirubin, mmol/L | 35 (26-50) | 26 (21-46) | .750 |
| Fetal hemoglobin, % | 13.2 (9.3-16.7) | 12.1 (9.0-15.9) | .649 |
| Recorded on hydroxyurea† | |||
| White blood cell count, 109/L | 6.700 (5.300-8.600) | 8.600 (7.200-14.925) | .002 |
| Neutrophil count, 109/L | 2.950 (2.330-3.930) | 3.655 (2.667-7.937) | .039 |
| Platelet count, 109/L | 308.000 (236.000-390.000) | 463.000 (272.250-566.500) | .011 |
| Hemoglobin level, g/dL | 8.8 (8.1-9.2) | 8.1 (7.7-8.7) | .027 |
| Hematocrit, % | 24.7 (23.2-26.2) | 23.6 (22.1-25.3) | .239 |
| Mean corpuscular volume, fL | 96.0 (81.7-101.7) | 85.5 (82.2-96.8) | .262 |
| Reticulocyte count, 109/L | 190.500 (130.800-236.100) | 172.500 (105.350-370.100) | .305 |
| Lactate dehydrogenase, IU/L | 420 (338-518) | 480 (380-530) | .348 |
| Bilirubin, mmol/L | 22 (17-56) | 23 (17-41) | .428 |
| Fetal hemoglobin, % | 14.9 (9.5-21.4) | 12.2 (5.5-20.3) | .236 |
| Hydroxyurea dose, mean mg/kg/d (SD) | 25.1 (2.2) | 23.1 (0.6) | .001 |
| Blood parameters . | Patients placed on hydroxyurea (N = 46) . | ||
|---|---|---|---|
| No reversion (n = 33), median (Q1-Q3) . | Reversion (n = 13), median (Q1-Q3) . | P* . | |
| At baseline | |||
| White blood cell count, 109/L | 14.800 (12.700-17.700) | 18.200 (11.975-20.950) | .051 |
| Neutrophil count, 109/L | 4.860 (4.205-7.297) | 6.225 (4.325-9.407) | .04 |
| Platelet count, 109/L | 354.000 (311.250-422.750) | 315.000 (262.250-349.750) | .189 |
| Hemoglobin level, g/dL | 7.8 (7.3-8.6) | 7.4 (6.7-8.0) | .074 |
| Hematocrit, % | 22.6 (21.5-25.7) | 22.0 (20.6-23.7) | .142 |
| Mean corpuscular volume, fL | 79.0 (74.4-83.1) | 82.7 (78.7-87.4) | .073 |
| Reticulocyte count, 109/L | 297.000 (248.200-355.000) | 448.100 (317.880-524.750) | .007 |
| Lactate dehydrogenase, IU/L | 974 (631-1249) | 998 (611-1267) | .656 |
| Bilirubin, mmol/L | 35 (26-50) | 26 (21-46) | .750 |
| Fetal hemoglobin, % | 13.2 (9.3-16.7) | 12.1 (9.0-15.9) | .649 |
| Recorded on hydroxyurea† | |||
| White blood cell count, 109/L | 6.700 (5.300-8.600) | 8.600 (7.200-14.925) | .002 |
| Neutrophil count, 109/L | 2.950 (2.330-3.930) | 3.655 (2.667-7.937) | .039 |
| Platelet count, 109/L | 308.000 (236.000-390.000) | 463.000 (272.250-566.500) | .011 |
| Hemoglobin level, g/dL | 8.8 (8.1-9.2) | 8.1 (7.7-8.7) | .027 |
| Hematocrit, % | 24.7 (23.2-26.2) | 23.6 (22.1-25.3) | .239 |
| Mean corpuscular volume, fL | 96.0 (81.7-101.7) | 85.5 (82.2-96.8) | .262 |
| Reticulocyte count, 109/L | 190.500 (130.800-236.100) | 172.500 (105.350-370.100) | .305 |
| Lactate dehydrogenase, IU/L | 420 (338-518) | 480 (380-530) | .348 |
| Bilirubin, mmol/L | 22 (17-56) | 23 (17-41) | .428 |
| Fetal hemoglobin, % | 14.9 (9.5-21.4) | 12.2 (5.5-20.3) | .236 |
| Hydroxyurea dose, mean mg/kg/d (SD) | 25.1 (2.2) | 23.1 (0.6) | .001 |
Bold type denotes statistically significant.
Blood parameters during hydroxyurea therapy were recorded when possible after at least 6 mo of treatment. However, in the case of velocities reversion to abnormal velocities, blood parameters were recorded at reversion time.
Hydroxyurea doses and blood parameters recorded 6 months postinitiation of hydroxyurea therapy are also shown in Table 4. Multivariate Cox regression analysis, with leukocytes, platelets, hemoglobin, and hydroxyurea dose as variables, retained only leukocytes (HR, 1.25 per 1 × 109/L increase; 95% CI, 1.08-1.43; P = .002) and hydroxyurea dose (HR, 0.59 per 1 mg/kg per day increase; 95% CI, 0.36-0.96; P = .033) as significant and independent associated risk factors for reversion on hydroxyurea treatment.
Treatment, velocities, silent infarcts, and stenosis at last TCDI and MRI/MRA assessment in the 92 patients.
At the last TCDI assessment, velocities were still abnormal in 6 of 92 patients, conditional in 8, normal in 76, and inadequate in 2. Twenty-six patients had stenosis at the first MRA, whereas at the last MRA, stenosis was present in only 17 patients because stenosis had disappeared in 11 (7 on transfusion and 4 posttransplant) but appeared in 2 who had high-conditional velocities despite chronic transfusion. At the last MRI, only 1 patient with abnormal TCDI despite chronic transfusion developed silent infarcts on transfusion.
The 24 patients transplanted with a genoidentical sibling donor are still alive with no chronic graft-versus-host disease, and 23 of 24 had normalized velocities at the last TCDI. Of note, 4 patients who still had abnormal TCDI despite chronic transfusion quickly normalized velocities posttransplant (Figure 4). Only 1 girl transplanted 15 years ago never normalized velocities in the left MCA and has persistent stenosis, although she has not experienced any stroke despite stopping transfusion after transplant. Stenosis was present in 7 patients at the first MRA and present in 3 at the last MRA. No patient developed silent infarct posttransplant.
Velocities outcome after transplant in 4 patients who still had abnormal velocities on chronic transfusion. Velocities were rapidly normalized after transplant. HSCT, hematopoietic stem cell transplant; l.MCA, left MCA; r.MCA, right MCA.
Velocities outcome after transplant in 4 patients who still had abnormal velocities on chronic transfusion. Velocities were rapidly normalized after transplant. HSCT, hematopoietic stem cell transplant; l.MCA, left MCA; r.MCA, right MCA.
Among the 46 patients who received hydroxyurea treatment, only 1 had stenosis at hydroxyurea initiation, prescribed because of return to Africa. This patient came back to France because of abnormal TCDI recurrence and the availability of a genoidentical donor. She was successfully transplanted, and stenosis disappeared. Among the other 45 patients, 2 developed stenosis secondarily. The first one had inadequate MRA at hydroxyurea initiation and the second one had presence of stenosis at the first MRA that had disappeared on chronic transfusion but reappeared on hydroxyurea with abnormal TCDI reversion. No patient developed silent infarct on hydroxyurea.
Duration of different therapies.
In the 92 patients, the mean duration of the first course of transfusion after abnormal TCDI detection was 2.6 ± 1.7 years (range, 0.3-8.5 years), but because several patients had a reversion to abnormal TCDI after hydroxyurea treatment, the total duration of transfusion was 3.7 ± 2.9 years (range, 0.3-13.9 years); the mean duration of hydroxyurea therapy in the 46 patients was 3.4 ± 2.7 years (range, 0.3-11.3 years) and the mean follow-up posttransplant was 3.5 ± 2.8 years (range, 0.3-11.0 years).
The follow-up for the 92 patients between the date of abnormal TCDI occurrence and the date of last TCDI assessment provided 585 patient-years, with 342 (61.1%) on transfusion, 8.4 with no treatment, 84.5 posttransplant, and 159.6 (28.5%) on hydroxyurea, considering the transfusion and hydroxyurea overlap during several months before transfusion cessation. Thus, transplant and hydroxyurea allowed reduction of chronic transfusion by 243 patient-years.
Discussion
Here, we report the outcome for 92 patients placed on transfusion at diagnosis of abnormal TCDI, including 45 patients switched to hydroxyurea and 24 transplanted, with a prolonged mean TCDI follow-up of 6.1 years. It is to be noted that the study used TCDI to estimate velocities, but that the velocity thresholds were those established with nonimaging TCD by the STOP trial. In France, TCDI is preferentially used instead of TCD because examinations are performed mainly in imaging departments that are equipped with color Doppler ultrasound machines and because TCDI is easier and faster to learn. Moreover, use of a color imaging ultrasound machine allows assessment of the extracranial part of the ICA, which is now done routinely at our center. Several studies reported that TCDI velocities are approximately 10% lower than those obtained by TCD,26-29 but also that differences decrease with sonographers’ experience and optimization of the technique.27 Others did not find any differences between TCD and TCDI.7 Thus, the French National Authority for Health decided that the same thresholds as those defined in the STOP study should be used30 to reduce any potential risk of patient “overtransfusion.” In the present study, patients with abnormal velocities in ACA or eICA (≥200 cm/s) were also treated with transfusion, contrary to the STOP-1 study wherein only abnormal velocities in MCA and ICA were treated with transfusion.8 Several reasons justify applying the same prevention for all arteries: normal ACA and eICA velocities are lower than MCA and ICA velocities,6,10,31 making ACA and eICA velocities ≥200 cm/s particularly high; high ACA velocities are associated with increased stroke risk31 ; and high eICA velocities are associated with stenosis and silent stroke risk.23
Normalization of velocities was observed in 76 of 91 patients (83.5%) placed on transfusion after a mean transfusion duration of 1.4 years. In the TCD with Transfusions Changing to Hydroxyurea (TWiTCH) trial,32 77% had normalized velocities at enrollment after a mean duration of transfusion of 4.3 years. The slight difference observed in the proportion of patients with velocity normalization in the TWiTCH trial compared to our cohort may be explained by the earlier detection of abnormal TCDI in our newborn cohort (mean age, 5.5 ± 2.0 vs 3.7 ± 1.5 years, respectively), suggesting that the earlier the abnormal velocities are detected, the higher the chances are that they will return to normal with transfusion. Kwiatkowski et al reported that patients who normalized velocities were younger at detection of abnormal TCDI than those who did not normalize (8.6 ± 4 vs 9.9 ± 3.7 years; P = .029).33 However, there was no age difference between the 2 groups in our newborn cohort (3.8 ± 1.5 vs 3.5 ± 1.8 years).
The data show that stenosis presence on MRA was a major risk factor for nonnormalization of velocities, increasing the risk by a factor of 2.6 in the multivariate Cox analysis. The probability of normalization was associated with lower velocities, as found in another study,33 but multivariate analysis including velocities and stenosis retained only the presence of stenosis as a significant risk factor. In the STOP-2 study,34 baseline MRA showed intracranial stenosis in 25 of 100 assessed patients (25%) before transfusion initiation. In our study, intracranial stenosis at the first MRA after abnormal TCDI occurrence was present in 19.8% of patients, a slightly lower percentage that may reflect the earlier detection of abnormal TCDI in this newborn cohort assessed as early as 12 to 18 months of age.
Considering the risk of severe side effects associated with chronic transfusion and the steady improvement with time of the results of transplant with genoidentical donors, familial HLA typing was performed and transplant was discussed with parents. We reported earlier21 that patients transplanted since the year 2000 had a 95% chance of cure, a percentage subsequently confirmed in a French series,35 with about a 3% risk of mortality. In the present cohort, 24 of 92 patients were transplanted. Donor engraftment was successful, and chronic transfusion was stopped in all patients. Moreover, rapid normalization of velocities occurred in 4 patients who had abnormal velocities before transplant despite chronic transfusion. These excellent results led us to initiate a prospective national trial (Drepagreffe, www.clinicaltrials.gov, #NCT01340404) in patients with a history of abnormal TCDI from centers throughout France, comparing the cerebrovascular outcome on transfusion or after transplant,36 and we are awaiting the final results.
Hydroxyurea, known to improve anemia and to decrease hemolysis16,17,25 and velocities,18,19,37 was an interesting candidate to replace transfusions in patients with a history of abnormal TCDI because we had shown that anemia, hemolysis, absence of α-thalassemia, and glucose-6-phosphate dehydrogenase deficiency were independent significant risk factors for occurrence of abnormal velocities.38 In the present long-term cohort study, hydroxyurea therapy was initiated in 1998, and 46 patients with a history of abnormal TCDI were treated, providing 160 patient-years on hydroxyurea since abnormal TCDI detection. Reversions were observed in 13 of 46 patients (28.3%), and transfusion was quickly re-initiated, emphasizing the necessity of a strict trimestrial follow-up by TCDI after hydroxyurea initiation in these patients with a history of abnormal velocities. However, hydroxyurea was successfully re-introduced in 6 of these patients after new normalization on chronic transfusion. The KM estimate of the proportion of patients with abnormal TCDI reversion on hydroxyurea at 1 year was 19.2% (95% CI, 7-31.4), which is lower than the 45% probability of events (reversion plus stroke) observed in the transfusion-halted arm of the STOP-2 trial,14 suggesting a favorable effect of hydroxyurea on reversion risk. We did not measure hydroxyurea levels in this study, preventing us from verifying treatment adherence, although nonadherence was highly suspected in 1 patient who experienced abnormal TCDI reversion. The results reported here argue for the safety of the switch to hydroxyurea because no stroke or silent infarct had occurred by the end of follow-up and only 1 patient developed recurrence of stenosis. Therefore, this procedure has economized 244 patient-years on chronic transfusion (about 2923 transfusions) without adding stroke risk to patients. We have identified that a high reticulocyte count >400 × 109/L recorded at baseline before age 3 years is highly predictive for reversion on hydroxyurea, multiplying the risk by a factor of 6.3. However, such data may not be observed because physicians may choose to initiate hydroxyurea therapy as soon as 9 months, independently of the severity of the disease. After hydroxyurea initiation, associated risk factors for reversion were high leukocyte count and low hydroxyurea dose, suggesting that reaching the MTD of hydroxyurea before stopping transfusion is important. Hydroxyurea therapy was initially promising for secondary stroke prevention,39 and the Stroke with Transfusions Changing to Hydroxyurea trial24,40 (2006-2010) randomized patients with a stroke history to either pursuing chronic transfusion with iron chelation or hydroxyurea with phlebotomy. This trial had a composite primary end point, and the study was prematurely closed because of equivalent iron content between the two arms, with no strokes in the transfusion/chelation arm but 7 strokes (10%) in the hydroxyurea/phlebotomy arm, despite being within the noninferiority stroke margin. It was concluded that transfusion/chelation remains the best way to manage children with SCA and stroke.24 The next study, TWiTCH,32,41,42 was a multicenter noninferiority trial in patients with a history of abnormal TCD and on chronic transfusion for at least 12 months who were randomized to transfusion pursuing vs hydroxyurea treatment. Started in 2011, it was prematurely closed in November 201441 because the primary end point had been reached and the strength of the statistical findings was unlikely to change with the collection of additional data. The hydroxyurea arm (n = 60) included an overlap period of 7 ± 2 months with transfusions until a stable MTD of hydroxyurea was reached. The primary study end point was the 24-month TCD velocity obtained from a linear mixed model, controlling for baseline (enrollment) values, with a noninferiority margin of 15 cm/s.42 By intention-to-treat analysis, the final calculated TCD velocities were similar in both arms, with the P value for noninferiority highly significant, resulting in the claim by the authors that hydroxyurea at MTD was noninferior and possibly superior to chronic transfusions for maintaining TCD velocities. The TWiTCH trial differs from our cohort study in several ways. In our cohort, all patients with reversion to abnormal velocities were treated with chronic transfusion as recommended by the STOP studies, and only patients with normalized velocities and no stenosis were switched to hydroxyurea, whereas in the TWiTCH trial, several patients still had mild stenosis and conditional or abnormal velocities at the time of the switch. It is interesting to note that despite this, no stroke occurred in the TWiTCH study, although this may be related to the very short follow-up without transfusion. In view of these data, it may be possible to extend our indications for the transfusion-to-hydroxyurea switch to those patients with mild stenosis or conditional velocities, although we would still recommend regular control by TCD/TCDI and MRI/MRA to re-introduce transfusions in the case of reversion or worsening vasculopathy. Another way our study differs from the TWiTCH study is that hydroxyurea initiation occurred after a shorter time on chronic transfusion (2.7 ± 1.7 vs 4.3 ± 2.4 years) and at an earlier age (6.4 ± 1.9 vs 9.8 ± 2.8 years) in our cohort. Because the oldest age for reversion was 9.5 years in our study, it is highly probable that most of the patients in the TWiTCH trial were away from the risk period. In addition, hydroxyurea MTD was reached in the TWiTCH trial, which may not have always been the case in our cohort, and may explain some reversions. Lastly, we think that the noninferiority in the TWiTCH trial may be due to the results of the TCD velocities being calculated using all TCD velocities captured throughout the trial, even during transfusion overlap, and to the short follow-up because of the premature closing and the intent-to-treat analysis. Adams et al in an ancillary study43 of the STOP trial reported the occurrence of strokes in 3 patients less than a month after TCD, with velocities of 202, 210, and 218 cm/s. Moreover, in the post-STOP-1 trial follow-up,44 all the 6 new subjects who developed stroke had an interpretable abnormal TCD prior to stroke. These data support our practice of quickly re-initiating transfusions in patients with reversion. Because early TCD screening and chronic transfusion have been proved to significantly reduce the risk of strokes,10 stopping transfusion in patients with abnormal velocities in countries where chronic transfusion is available should be exercised with caution. Furthermore, a subset of patients had persistent abnormal TCD on transfusion therapy. Kwiatkowski et al in a substudy of the STOP trial reported33 no stroke occurrence among 19 patients with abnormal TCD after a mean follow-up of 2.4 years on transfusion therapy; however, MRI/MRA was not routinely performed then. In contrast, strokes occurred in 2 patients with abnormal TCD on transfusions in the post-STOP-1 trial follow-up.44 In our study, no stroke occurred despite the persistence under transfusion therapy of abnormal TCD in 6 patients and conditional velocities in 8, whereas 2 developed stenosis and 1 had a silent infarct. These data suggest a worsening vasculopathy in patients without normalized velocities, supporting the use of alternative therapies such as transplant while evaluating the risks/benefits of the procedures for each patient.
Our study has some limitations because it is a longitudinal prospective observational cohort study with no control group. Thus, effectiveness or net benefits cannot be inferred here. Only prospective randomized trials in patients with a history of abnormal velocities comparing hydroxyurea to transfusion such as the TWiTCH trial37 or a trial comparing transplantation to transfusion such as the French Drepagreffe trial36 could answer these questions. Nevertheless, this study offers the advantages of long-term and accurate data collection in a cohort from a single referral center where management care and procedures were carefully adopted and standardized. The mean duration of hydroxyurea therapy in our cohort was 3.4 ± 2.7 years, which is longer than the TWiTCH follow-up, initially scheduled for 2 years for each patient but stopped early. Similarly, the planned follow-up of patients in the Drepagreffe trial36 after the randomization is for 1 year, whereas the mean follow-up posttransplant in the present cohort is 3.5 ± 2.8 years. Thus, the long follow-up in our cohort gives a better opportunity to evaluate the risk of reversions to abnormal TCDI after transfusion discontinuation.
We conclude that in patients with a history of abnormal velocities, discontinuation of chronic transfusion with the switch to hydroxyurea can be safe in a subset of patients with normalized velocities and no stenosis, provided that the patients be reassessed with Doppler each trimester and transfusions be re-initiated as soon as abnormal velocities return. However, transplant with a genoidentical donor remains the only procedure allowing the safe cessation of transfusions in all patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, their parents, and all the nurses and physicians at CHIC who contributed to the management of patient care, and Martine Torres for her critical reading of the manuscript and editorial assistance.
This work was supported in part by the institutional grant “Programme Hospitalier de Recherche Clinique” (IDF05001) from the French Ministry of Health.
Authorship
Contribution: F.B. designed and performed the research, collected the data, performed the statistical analyses, interpreted the data, and wrote the manuscript; S.V. designed the study, performed the Doppler ultrasound scans and MRI/MRA, analyzed and interpreted the data, and cowrote the manuscript; C.P., C.A., and A.K. designed the study, collected and interpreted the data, and cowrote the manuscript; F.B., C.P., C.A., A.K., I.H., F.M., C.F., S.B., E.L., and R.E. participated in the management of patient care; M.V. and F.K. performed the Doppler ultrasound scans and MRI/MRA. E.G., J.-H.D., and G.S. performed the transplants. All authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Bernaudin, Pédiatrie, Centre de Référence des Syndromes Drépanocytaires Majeurs, Centre Hospitalier Intercommunal de Créteil, 40 Ave de Verdun, 94010 Créteil, France; e-mail: francoise.bernaudin@chicreteil.fr.