In this issue of Blood, Bernaudin et al report that chronic red blood cell transfusions can be safely replaced with hydroxyurea therapy or bone marrow transplantation for a cohort of children with sickle cell anemia (SCA) and abnormal transcranial Doppler (TCD) velocities.1 These data nicely complement the recently published results from the phase 3 multicenter TCD With Transfusions Changing to Hydroxyurea (TWiTCH) study and suggest that it may be safe to carefully transition a subset of patients from chronic transfusions to hydroxyurea therapy.2
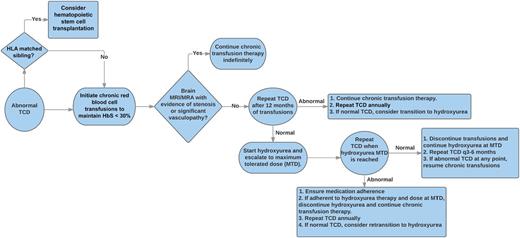
This proposed algorithm provides guidance for the management of children with abnormal TCD velocities, including decision points regarding the initiation or discontinuation of chronic blood transfusions and hydroxyurea therapy. HbS, sickle hemoglobin; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
This proposed algorithm provides guidance for the management of children with abnormal TCD velocities, including decision points regarding the initiation or discontinuation of chronic blood transfusions and hydroxyurea therapy. HbS, sickle hemoglobin; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
Over the last 20 years, we have seen dramatic reductions in the frequency of stroke for children with SCA. Prior to active surveillance with TCD and routine use of either chronic blood transfusions or hydroxyurea therapy, the Cooperative Study of Sickle Cell Disease demonstrated that 11% of children with SCA will suffer a stroke before 20 years of age.3 At that time, there were no screening tests to identify those at highest risk and treatment included supportive care once a stroke did occur. At that point, unfortunately, the damage had been done. In the 1990s, we learned that TCD velocities can be used to identify children at high risk for stroke, and the subsequent Stroke Prevention Trial in Sickle Cell Anemia (STOP) clearly demonstrated that chronic transfusion therapy reduces the risk of stroke for children with abnormal TCD velocities.4,5 This work led to a paradigm shift in the standard of care to now include screening TCDs for all children with SCA and chronic blood transfusion programs as primary stroke prophylaxis for those at highest risk. This aggressive screening and preventive approach has resulted in significant reductions in the frequency of stroke.6
Although the benefits of TCD screening and chronic blood transfusions for primary stroke prevention are indisputable, they do not come without cost, both literally and figuratively. If a child has abnormal TCD velocities at age 2 years of age, current guidelines would suggest that this child receive monthly blood transfusions indefinitely. Chronic blood transfusion therapy requires prolonged monthly hospital visits, costly iron chelation therapy, and oftentimes surgical procedures to implant central venous catheters. In addition to the cost and inconvenience, chronic transfusion therapy is associated with serious and life-threatening hemosiderosis, most commonly in the liver, as well as the development of auto- or alloantibodies to erythrocyte antigens that can make it difficult to find compatible blood. For these reasons, in combination with the emergence of hydroxyurea therapy as a widely available and safe disease-modifying therapy, there has been great interest in identifying safe alternatives to chronic transfusion therapy with improved iron management, particularly for the prevention and treatment of the neurologic consequences of SCA.
The current report by Bernaudin et al carefully demonstrates that there are alternatives to chronic transfusion therapy for children with abnormal TCD velocities and no significant intracranial stenosis. The authors report normalization of TCD velocities in 23 of 24 patients who received bone marrow transplantation with a matched sibling donor, and all transplanted patients survived transplant without any reported long-term toxicities. Forty-six children were transitioned to hydroxyurea therapy, with the majority of patients doing well with normalization of TCD velocities and no reported serious neurologic events. These data are a timely parallel to the recently published results from TWiTCH, which was a multicenter, open-label, phase 3, noninferiority trial, in which children with abnormal TCDs were transitioned to hydroxyurea therapy after ≥1 year of chronic transfusion therapy. The study was halted early when hydroxyurea was found to be noninferior to chronic transfusion therapy as defined by the study’s primary end point: 24-month TCD velocity. The patient population for TWiTCH was carefully selected, and the process of hydroxyurea dosing and monitoring was rigorous. It is therefore important to validate these results in a more practical setting and the current report does just that. Although this French cohort is particularly well cared for and carefully monitored by a very experienced clinical research team, the current report was not in the context of a rigorous randomized clinical trial and thus represents early “effectiveness” data to indicate that there are safe alternatives to lifelong chronic transfusion therapy.
While certainly encouraging, these findings are not yet a “slam dunk” to suggest that hydroxyurea is now a universal replacement for chronic transfusions to prevent neurologic complications for children with SCA. Thirteen of the 36 patients transitioned to hydroxyurea demonstrated reversion to abnormal TCD velocities, although 4 occurred within the first 6 months of hydroxyurea therapy before maximum tolerated dose had been reached. Once an abnormal TCD was identified, the patients were again placed on chronic transfusions, and, ultimately, 6 of 13 were transplanted and 4 of 13 were able to be successfully transitioned a second time back to hydroxyurea therapy with normalization of TCD velocities. These carefully documented observations provide important data to suggest that a combination of therapies with careful surveillance may be a safe and effective alternative to indefinite chronic transfusions alone. The figure provides a potential clinical algorithm for the short- and long-term management of children with abnormal TCD velocities.
With easily available TCD and MRI/MRA studies and multimodal disease-modifying therapy starting early in life, we continue to see changes with the natural history of SCA and are moving toward the goal of a stroke-free childhood for patients with SCA. The early initiation of hydroxyurea therapy for infants with SCA is likely to reduce the number of children who develop abnormal TCD velocities in the first place, and a careful treatment and follow-up strategy for children with abnormal velocities will further reduce the frequency and severity of neurologic complications for children with SCA. It will be critical to further validate these results to determine whether it truly is safe to “flip the switch” from chronic transfusions to hydroxyurea, particularly in the context of the real-world clinical challenges around medication adherence, appropriate dose escalation, and timely monitoring and follow-up.
Conflict-of-interest disclosure: The author declares no competing financial interests.