In this issue of Blood, Santagostino et al, in their phase 3 study, demonstrate efficacy and safety of recombinant fusion protein linking coagulation factor IX (FIX) with albumin (rIX-FP) which, along with the other 2 extended half-life FIX products, heralds a new era for the treatment of hemophilia B.1
We are in an era of safe effective treatment of hemophilia. Since the late 1980s, HIV and hepatitis C virus have been eliminated from plasma-derived factor products. Recombinant products have been available for hemophilia B for over 20 years. Prophylactic treatment to prevent bleeding has become standard of care.2 Before 2014, the half-life of available FIX concentrates was around 18 to 27 hours,3,4 thus necessitating factor infusion about twice weekly to sustain FIX trough levels above 1% and therefore decreasing the chance of spontaneous bleeding. Different technologies have emerged to prolong the half-life of FIX concentrate. The first extended half-life factor utilizing the neonatal Fc receptor (recombinant FIX [rFIX] Fc fusion protein [rFIXFc])5 was US Food and Drug Administration (FDA) approved in March 2014. Results of a second protein, a recombinant glycoPEGylated FIX (nonacog β pegol), were reported by Collins et al.6 This trial by Santagostino and colleagues investigates a rIX-FP and completes the last of 3 first-generation recombinant extended half-life FIX phase 3 studies (see table).7 These products have the potential to revolutionize hemophilia treatment with dosing intervals increasing from twice a week to dosing every 7 to 21 days and increasing FIX trough levels.
Characteristics of 3 extended half-life FIX concentrates from recently completed phase 3 clinical studies
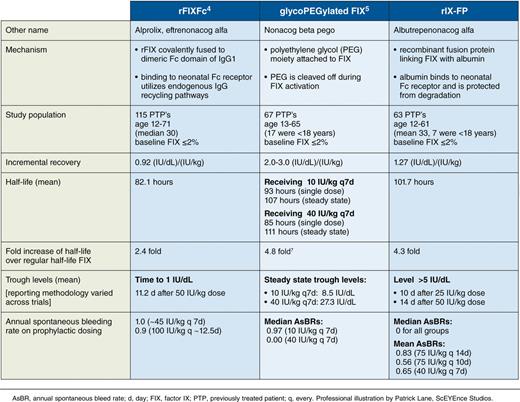
The open-label phase 3 study presented in this issue demonstrates efficacy of rIX-FP to prevent bleeding episodes. rIX-FP is a recombinant fusion protein linking FIX with recombinant albumin. It utilizes the neonatal Fc receptor to recycle the factor after endocytosis,8 a technology that has been shown to be safe and effective in other settings.9 This modification results in a 4.3-fold increase in factor half-life as compared with a traditional recombinant FIX product (rFIX), equivalent to a half-life of 102 hours in this study. Weekly injections of 40 IU/kg resulted in a trough rIX-FP of 20% whereas every 14-day injections resulted in a trough of 12%. Like most modern factor studies, subjects had low bleeding rates and the treatment was well tolerated with a median annualized bleeding rate of 0.0. Furthermore, 99% of bleeding episodes were successfully treated with FIX-FP, 94% of bleeds with only a single dose. In this study of previously treated patients, no FIX inhibitors were detected throughout the study. It is noteworthy that subjects in the on-demand arm had a 100% reduction in annualized spontaneous bleeding and full resolution of target joints.
An interesting facet to this study was the flexibility given to the treating physician. Subjects in the prophylaxis group received 35 to 50 IU/kg weekly. The exact entry dose was determined by the treating physician. If there was no spontaneous bleeding in the first 26 weeks on this weekly dosing, dose and dosing interval could be adjusted to 75 IU/kg every 10 to 14 days. Importantly, the physician could increase or decrease the dose received based on clinical assessment. This flexibility gave the study a real-life feel, allowing physicians to make clinical judgments. Conceivably, this real-world design could ease the application in clinical use.
One of the vexing problems with all chronic illnesses is patient treatment fatigue. Decreasing dosing intervals may improve this issue in hemophilia B, increase adherence to prophylactic treatment, and prevent bleeding and associated joint arthropathy. Another key element of extended half-life rFIX products is the potential for higher trough levels which decrease the risk for breakthrough bleeding. A desirable trough level in the past has been considered to be >1% due to the notion that, by converting someone with severe disease to a phenotypic moderate hemophilia, spontaneous bleeding could be prevented. With the advent of these longer-acting FIX products, aiming for a higher trough level has become feasible and we have to ask whether 1% is enough for our patients with hemophilia. People with moderate and mild hemophilia (FIX levels of 2%-30%) still potentially experience microbleeding and certainly trauma/activity-related bleeding that can lead to undesirable outcomes. Combining increased compliancy with higher trough level allows for the normalization of activity and increasing long-term joint and overall health. The economic impact of this new paradigm certainly has to be considered. We are truly entering a new era for hemophilia B treatment.
Conflict-of-interest disclosure: J.A.T. is on advisory committees for Biogen, CSL-Behring, and Baxalta, and is receiving investigator-initiated funding from Biogen, Kedrion, Novo Nordisk, and Baxalta. R.K.-J. has been a paid consultant for Baxalta, Bayer, CSL-Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, and Roche, and is receiving research funding from Pfizer and Roche.
