To the editor:
We have read with interest the letter by Baliakas et al1 on the impact of MYD88 mutation in IGHV mutated (M-IGHV) chronic lymphocytic leukemia (CLL) patients. They reported a frequency of MYD88-mutated patients of 4% in a series of 558 M-IGHV CLL, and found that M-IGHV CLL patients with mutated MYD88 were predominantly male, had a similar age at diagnosis, and presented in a more advanced clinical stage, with mainly favorable cytogenetic abnormalities and a lower frequency of TP53, SF3B1, or NOTCH1 mutations than MYD88 unmutated patients. Additionally, the authors found a trend to a shorter time to first treatment (TTFT) in MYD88-mutated patients due to the higher frequency of advanced clinical stage. Then, they compared their results with our previously published series,2 concluding that they could not confirm the young age and the good outcome of the patients with MYD88 mutations observed in our study.
We would like to clarify some aspects that apparently are different in both studies. First, we are afraid that the authors misunderstood some of our results because, in contrast to what they stated in their letter, we did not find differences in Binet stage A and B TTFT according to MYD88 mutations, neither in the whole series nor in patients <50 years (see Tables 1 and 2 in our paper).2 In fact, our results were identical to theirs. Time to next treatment (TTNT) was the end point we found significantly longer in MYD88/TLR-mutated patients with respect to wild-type cases. However, this parameter was not studied by Baliakas et al. In our opinion, the difference between both studies may be related to some differences in the design of the studies that may explain the apparently contradictory results. Their study included only IGHV-mutated CLL, whereas ours analyzed unselected CLL patients. Conversely, Baliakas et al studied basically the L265P MYD88 mutation, whereas we investigated several genes of the TLR/MYD88 pathway. Balaikas et al examined only exon 5 of MYD88 in 1039 patients and exon 3 in 402 of them, whereas our study investigated several genes of the TLR/MYD88 pathway in 587 patients by whole exome sequencing (41% of cases) or Sanger analysis, which in addition to MYD88, included IRAK1, TLR2, TLR5, and TLR6. Mutations in genes of the MYD88/TLR pathway other than the MYD88 L265P hot spot constituted 30% of the total mutations in our series (MYD88 mutations outside exon 5 in 3 cases and in other genes of TLR pathway in 4 cases). Finally, Baliakas et al did not observe a relationship between MYD88 mutations and the younger age of the patients as we did. However, in our series, 25% of the patients were <50 years, whereas the relative number of young patients in the cohort of Baliakas et al was not defined. We could postulate that the number of young patients in their series may be low and perhaps not sufficient to capture this feature. The median age was lower in our cohort than in that of Baliakas et al (61 vs 65 years), and a high frequency of MYD88 mutations (8-10%) has also been observed in other studies with younger median age3,4 compared with those with older median age.5,6
We agree with Baliakas et al that, given the heterogeneity of CLL, there are still many important unsolved issues concerning the recurrently mutated genes and their relationship with other prognostic factors. Following their suggestion,1 we have reanalyzed our cohort focusing only on M-IGHV cases, with the addition of 55 new patients with M-IGHV (total 311 patients). We also expanded the follow-up of our patients up to July 2015. We have information of the whole MYD88 in 200 cases and of MYD88 exon 5 in 111 cases. We found MYD88 mutations in 23 of 311 (7.4%) patients: 16 (70%) were the L265P variant and 7 (30%) were other MYD88 mutations (2 cases with V247F, 2 cases M232T, and 1 case each of V147L, S219C, and S243N). In agreement with Baliakas et al,1 we observed similar characteristics among M-IGHV CLL irrespective of MYD88 mutations (Table 1). In addition, we confirmed that MYD88-mutated patients were significantly younger (median age, 50 vs 61 years; P = .001), had a trend toward male predominance, had a lower frequency of Binet stage A (77% vs 94%; P = .02), had an almost absence of adverse cytogenetic alterations or mutations in TP53, NOTCH1, SF3B1, ATM, and BIRC3, and had a lower percentage of cases with high expression of CD38 (0% vs 16%; P = .032) than in patients with wild-type MYD88.
Main clinical characteristics and outcome of M-IGVH CLL according MYD88 mutational status
| Parameter . | Category . | MYD 88 mutated (n = 23) . | MYD88 unmutated (n = 288) . | P . |
|---|---|---|---|---|
| Sex | Male (%) | 17/23 (74%) | 163/288 (57%) | .14 |
| Age (years), median (range) | 50 (32-73) | 61 (29-89) | <.001 | |
| Binet stage | A | 17 (77%) | 269 (94%) | |
| B | 3 (13%) | 14 (5%) | .008 | |
| C | 2 (9%) | 4 (1%) | ||
| Rai stage | 0 | 9 (41%) | 207 (72%) | |
| I-II | 11 (50%) | 77 (27%) | .002 | |
| III-IV | 2 (9%) | 4 (1%) | ||
| CD38 | >30% | 0/23 (0%) | 43/268 (16%) | .032 |
| ZAP-70 | >20% | 1/23 (4.3%) | 20/258 (8%) | NS |
| Genetics | Del(13q) | 10/15 (66%) | 118/220 (54%) | NS |
| Trisomy 12 | 2/15 (13%) | 22/221 (10%) | NS | |
| Del(11q) | 0/15 (0%) | 5/220 (2%) | NS | |
| Del(17p) | 0/15 (0%) | 5/220 (2%) | NS | |
| NOTCH1 | Mutated | 0/19 (0%) | 8/256 (3%) | NS |
| SF3B1 | Mutated | 0 /17 (0%) | 14/233 (6%) | NS |
| BIRC3 | Mutated | 0/18 (0%) | 6/188 (3%) | NS |
| ATM | Mutated | 0/18 (0%) | 7/188 (4%) | NS |
| TP53 | Mutated | 1/20 (5%) | 10/217 (5%) | NS |
| Drivers (mean, SD) | 2.6 ± 1.2 | 1.7 ± 1.3 | .007 | |
| Treated patients | 12/23 (52%) | 99/285 (35%) | .07 | |
| 10-year TTFT (95% CI) | Binet A and B | 38% (12-64) | 35% (29-41) | NS |
| 10-year TTFT (95% CI) | Binet A | 27% (1-54) | 33% (27-39) | NS |
| 10-year OS (95% CI) | All | 100% | 85% (80-90) | .047 |
| Parameter . | Category . | MYD 88 mutated (n = 23) . | MYD88 unmutated (n = 288) . | P . |
|---|---|---|---|---|
| Sex | Male (%) | 17/23 (74%) | 163/288 (57%) | .14 |
| Age (years), median (range) | 50 (32-73) | 61 (29-89) | <.001 | |
| Binet stage | A | 17 (77%) | 269 (94%) | |
| B | 3 (13%) | 14 (5%) | .008 | |
| C | 2 (9%) | 4 (1%) | ||
| Rai stage | 0 | 9 (41%) | 207 (72%) | |
| I-II | 11 (50%) | 77 (27%) | .002 | |
| III-IV | 2 (9%) | 4 (1%) | ||
| CD38 | >30% | 0/23 (0%) | 43/268 (16%) | .032 |
| ZAP-70 | >20% | 1/23 (4.3%) | 20/258 (8%) | NS |
| Genetics | Del(13q) | 10/15 (66%) | 118/220 (54%) | NS |
| Trisomy 12 | 2/15 (13%) | 22/221 (10%) | NS | |
| Del(11q) | 0/15 (0%) | 5/220 (2%) | NS | |
| Del(17p) | 0/15 (0%) | 5/220 (2%) | NS | |
| NOTCH1 | Mutated | 0/19 (0%) | 8/256 (3%) | NS |
| SF3B1 | Mutated | 0 /17 (0%) | 14/233 (6%) | NS |
| BIRC3 | Mutated | 0/18 (0%) | 6/188 (3%) | NS |
| ATM | Mutated | 0/18 (0%) | 7/188 (4%) | NS |
| TP53 | Mutated | 1/20 (5%) | 10/217 (5%) | NS |
| Drivers (mean, SD) | 2.6 ± 1.2 | 1.7 ± 1.3 | .007 | |
| Treated patients | 12/23 (52%) | 99/285 (35%) | .07 | |
| 10-year TTFT (95% CI) | Binet A and B | 38% (12-64) | 35% (29-41) | NS |
| 10-year TTFT (95% CI) | Binet A | 27% (1-54) | 33% (27-39) | NS |
| 10-year OS (95% CI) | All | 100% | 85% (80-90) | .047 |
CI, confidence of interval; NS, not significant; SD, standard deviation.
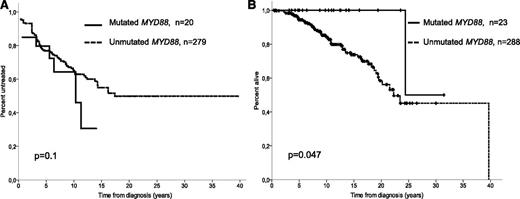
As seen in Table 1 and Figure 1A, 10-year TTFT in Binet A and B patients did not differ between both groups (36% vs 35%, respectively; P = .1), as previously reported.2 The same is true if only Binet A stage patients are considered (10-year TTFT: 24% vs 33% for MYD88-mutated and wild type, respectively; P = .65). Of the 88 patients achieving a complete response or partial response, 42 eventually required a second treatment. MYD88-mutated patients showed a significantly longer TTNT (5-year retreated patients: 45% in wild-type MYD88 CLL vs 0% in MYD88-mutated CLL; P = .05). The 10-year overall survival (OS) of M-IGHV CLL patients with MYD88 mutations showed a significant better OS than wild-type MYD88 (10-year actuarial probability: 100% vs 85%; P = .047; Figure 1B), in agreement with our previous results. Finally, we confirmed the noninferior life expectancy for patients with MYD88-mutated CLL compared with the sex- and age-matched general population.
Outcome of M-IGHV CLL patients according to the mutational status of MYD88. Actuarial curves for (A) time to first treatment in Binet stages A and B and (B) overall survival of mutant (solid line) and wild-type (dashed line) MYD88 CLL patients with mutated IGHV.
Outcome of M-IGHV CLL patients according to the mutational status of MYD88. Actuarial curves for (A) time to first treatment in Binet stages A and B and (B) overall survival of mutant (solid line) and wild-type (dashed line) MYD88 CLL patients with mutated IGHV.
Several gene mutations found in CLL are highly associated with 1 of the 2 IGHV mutational groups.2,3,7-9 In our series, MYD88 mutations are almost exclusively found in M-IGHV CLL (23 of 24 cases, 96%). Thus, a comparison of clinico-biological characteristics of this type of mutation inside their specifically associated IGHV mutational group could provide a more precise insight in the prognostic impact of that type of mutations in CLL patients. Moreover, cooperative studies for assessing the prognostic role of this and other low-frequency mutations in larger series of CLL patients are warranted.
Authorship
Contribution: A.M.-T., J.D., and A.L.-G. reviewed clinical data; A.N. performed the mutational analysis; M.A. prepared and supervised the bioethics requirements; E.C. and N.V. reviewed the pathologic data and confirmed the diagnosis; A.L.G., E.C., and N.V. directed the research; A.M.-T., A.L.-G., and N.V. wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neus Villamor, Unitat d'Hematopatogia, Hospital Clínic, Carrer Villarroel 170, 08036 Barcelona, Spain; e-mail: villamor@clinic.cat.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal