Key Points
The impaired suppressive function of myeloid-derived suppressor cells plays a role in the pathogenesis of immune thrombocytopenia.
The effect of dexamethasone in correcting dysfunction of myeloid-derived suppressor cells suggests a new therapeutic mechanism of high-dose dexamethasone in patients with immune thrombocytopenia.
Abstract
Myeloid-derived suppressor cells (MDSCs) are heterogeneous immature cells and natural inhibitors of adaptive immunity. In this study, the MDSC population was evaluated in adult patients with primary immune thrombocytopenia (ITP), where cell-mediated immune mechanisms are involved in platelet destruction. Our data demonstrated that both the numbers and suppressive functions of MDSCs were impaired in the peripheral blood and spleens of patients with ITP compared with healthy control patients. High-dose dexamethasone (HD-DXM) treatment rescued MDSC numbers in patients with ITP. And DXM modulation promoted the suppressive function of MDSCs induced in vitro. Moreover, the expression of interleukin 10 and transforming growth factor β was significantly upregulated in DXM-modulated MDSCs compared with the unmodulated cultures. DXM-modulated MDSCs inhibited autologous CD4+ T-cell proliferation and significantly attenuated cytotoxic T lymphocyte–mediated platelet lysis, further indicating enhanced control over T-cell responses. Elevated expression of the transcription factor Ets1 was identified in DXM-modulated MDSCs. Transfection of Ets-1 small interfering RNA efficiently blocked regulatory effects of MDSCs, which almost offset the augmentation of MDSC function by DXM. Meanwhile, splenocytes from CD61 knockout mice immunized with CD61+ platelets were transferred into severe combined immunodeficient (SCID) mouse recipients (C57/B6 background) to induce a murine model of severe ITP. We passively transferred the DXM-modulated MDSCs induced from bone marrow of wild-type C57/B6 mice into the SCID mouse recipients, which significantly increased platelet counts in vivo compared with those receiving splenocyte engraftment alone. These findings suggested that impaired MDSCs are involved in the pathogenesis of ITP, and that HD-DXM corrected MDSC functions via a mechanism underlying glucocorticoid action and Ets1.
Introduction
Myeloid-derived suppressor cells (MDSCs) are heterogeneous cells characterized by a myeloid origin, immature state, and remarkable ability to suppress T-cell responses, which expands during cancer, inflammation, and infection.1 These cells circulate in the blood and accumulate in the lymphoid tissue, bone marrow, and especially tumor sites.2 T-cell inactivation can be mediated by MDSCs through interferon (IFN)-γ-dependent nitric oxide production or the Th2-mediated interleukin (IL) 4/IL-13-dependent Arginase-1 (Arg-1) pathway.3-7 l-arginine deprivation results in repressed expression of the T-cell-signaling molecule, CD3ζ, and the arrest of T-cell cycle.8 Therefore, 2 enzymes that compete for l-arginine metabolism (Arg-1 and inducible nitric-oxide synthase [iNOS]) are crucial for MDSC-induced T-cell dysfunction.7,9 The above suppression mirrored the modulatory effect of T regulatory cells (Tregs), indicating that MDSC-induced immune tolerance may be synergistic with the recruitment and expansion of Tregs.10
In mice, MDSCs are generally characterized by the expression of cell surface antigens Ly-6C/G and CD11b, and their human counterparts are typically CD11b+CD33+HLA-DR–.11,12 Although these cells are posited to play deleterious roles in malignancies, functional MDSCs may be beneficial and protective in autoimmunity.13 Reports have demonstrated that myeloid cells could intrinsically suppress T-cell proliferation and maintain immune homeostasis in murine models of autoimmune diseases.14,15 However, the role of MDSCs in human autoimmunity remains obscure.
Immune thrombocytopenia (ITP) is an autoimmune hemorrhagic disorder in which enhanced anti-platelet T helper cell activity and abnormal activation of CD8+ T cells are involved.16-19 High-dose dexamethasone (HD-DXM) has been widely used as the first-line therapy for patients with ITP.20,21 Previous literature confirmed that a 4-day regimen of HD-DXM expanded both Tregs and myeloid dendritic cells (mDCs) in ITP, suggesting the immunosuppressive therapy with glucocorticoids (GCs) could restore regulatory cells in an autoimmune scenario.22 GCs have been found to induce a specific monocytic phenotype with anti-inflammatory properties in humans, and a corresponding subset of monocytes induced by GCs in mice has been proved to resemble tumor-derived MDSCs.23 Nonetheless, little has been defined about the effect of GCs on MDSCs in ITP, and whether this effect contributes to the remission of disease.
MDSCs have a gene expression profile distinct from that of normal monocytes and polymorphonuclear neutrophils,24 among which Ets1 is regarded as a potential factor responsible for various autoimmune diseases.25 In this study, by looking into the unique function of MDSCs, we investigated whether a comparative loss in number and suppressive activity of MDSCs had a place in the pathogenesis of ITP. Our data elucidated both in vitro and in vivo that HD-DXM restored the insufficiency of MDSCs in ITP and identified a role for DXM in correcting impaired MDSC function. We implicated that this effect might be achieved via a possible crosstalk between glucocorticoids receptor (GR) and the transcription factor Ets1. Our results provided novel insights into a possible mechanism of glucocorticoid strategy in the management of ITP.
Materials and methods
Patients and controls
Twenty-one patients with active primary ITP (11 women and 10 men; age range, 18-70 years; median age, 40 years; platelet count range, 2-25 × 109/L; median platelet count, 12 × 109/L) were enrolled between May 2013 and March 2015 (supplemental Table 1, available on the Blood Web site). The diagnostic criteria were consistent with previously reported consensus.18 Cases complicated by diabetes, hypertension, cardiovascular diseases, pregnancy, active infection, or connective tissue diseases were excluded. These 21 patients received HD-DXM 40 mg/d for 4 consecutive days, as described in supplemental Data. Eighteen healthy adult volunteers (9 women and 9 men; age range, 20-45 years; median age, 29 years; platelet count range, 165-275 × 109/L; median platelet count, 191 × 109/L) were recruited as control patients. Spleen specimens from 5 patients with ITP and 5 patients with traumatic spleen rupture were analyzed by immunofluorescence (supplemental Table 2). This study was approved by the Medical Ethics Committee of Qilu Hospital, Shandong University. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Mice
Wild-type (WT) C57BL/6J mice as platelet donors were obtained from the Center for New Drug Evaluation of Shandong University. Severe combined immunodeficient (SCID) mice of C57BL/6 background (J001913, 6-8 weeks of age) as spleen cell transfer recipients were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6 CD61-KO mice (B6.129S2-Itgb3tm1Hyn/JSemJ; Stock No: 008819) were provided by Dr. Junling Liu from Shanghai Jiaotong University School of Basic Medicine. All animal experiments were performed under the approval of the Animal Ethics Committee of Shandong University School of Medicine.
Fluorescence-activated cell sorter analysis
The phenotype of MDSCs was analyzed for the cell surface markers CD11b, CD33, and HLA-DR, as described in supplemental Data. Intracellular expression of Arg-1 and iNOS was also determined. Data acquisition was performed on a Gallios Flow Cytometer (Beckman Coulter). A total of 20 000 events were analyzed using Kaluza Flow Cytometry Analysis Software (Beckman Coulter).
Preparation of peripheral blood mononuclear cells and splenocytes
Whole blood was obtained from patients with ITP and healthy volunteers by venipuncture into ethylenediaminetetraacetic acid. Peripheral blood mononuclear cells (PBMCs) were isolated and resuspended at 5 × 105 cells/mL. Splenocytes were prepared and strained through a 200 mesh copper filter in phosphate-buffered saline. Cell suspension was centrifuged at 400 g for 15 minutes and adjusted to a concentration of 5 × 107 cells/mL.
In vitro generation of human MDSCs
Both PBMCs and splenocytes were cultured in complete medium for 7 days. Each culture was supplemented with recombinant human IL-6 (10 ng/mL; R&D Systems, Minneapolis, MN) and granulocyte macrophage–colony-stimulating factor (10 ng/mL; R&D Systems) in the presence or absence of DXM and incubated in humidified air with 5% CO2 at 37°C. Cells cultured in medium alone were run in parallel as controls. Adherent cells were removed using a nonprotease cell detachment solution Detachin (Neuromics, Edina, MN). CD33+ cells were isolated by anti-CD33 magnetic microbeads and LS column separation (Miltenyi Biotec, Bergisch Gladbach, Germany), per manufacturer’s instructions. The purity of the isolated cells was found to be higher than 90% by flow cytometry.
Quantitative real-time PCR
Total RNA was extracted and converted into cDNA, as described in supplemental Data, and run on a LightCycler 480 II (Roche, Basel, Switzerland). Primer sequences were obtained from the National Institutes of Health quantitative reverse-transcription polymerase chain reaction database (http://primerdepot.nci.nih.gov; Table 1).
Gene-specific primers for quantitative reverse-transcription polymerase chain reaction
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| ARG-1 | 5′-GTTTCTCAAGCAGACCAGCC-3′ | 5′-GCTCAAGTGCAGCAAAGAGA-3′ |
| iNOS | 5′-ATTCTGCTGCTTGCTGAGGT-3′ | 5′-TTCAAGACCAAATTCCACCAG-3′ |
| TGF-β | 5′-GCAGAAGTTGGCATGGTAGC-3′ | 5′-CCCTGGACACCAACTATTGC-3′ |
| VEGF | 5′-CACACAGGATGGCTTGAAGA-3′ | 5′-AGGGCAGAATCATCACGAAG-3′ |
| IL-10 | 5′-TCAAACTCACTCATGGCTTTGT-3′ | 5′-GCTGTCATCGATTTCTTCCC-3′ |
| COX2 | 5′-GTTTTGACATGGGTGGGAAC-3′ | 5′-CCCTCAGACAGCAAAGCCTA-3′ |
| CD33 | 5′-TTCCTCCTGTGGGTCTTCAC-3′ | 5′-CTTTCCAGGAGATGGCTCAG-3′ |
| ETS1-96 | 5′-GATAGTTGTGATCGCCTCACC-3′ | 5′-GTCCTCTGAGTCGAAGCTGTC-3′ |
| ETS1-237 | 5′-TACACAGGCAGTGGACCAATC-3′ | 5′-CCCCGCTGTCTTGTGGATG-3′ |
| GAPDH | 5′-CACACAGGATGGCTTGAAGA-3′ | 5′-AGGGCAGAATCATCACGAAG-3′ |
| Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| ARG-1 | 5′-GTTTCTCAAGCAGACCAGCC-3′ | 5′-GCTCAAGTGCAGCAAAGAGA-3′ |
| iNOS | 5′-ATTCTGCTGCTTGCTGAGGT-3′ | 5′-TTCAAGACCAAATTCCACCAG-3′ |
| TGF-β | 5′-GCAGAAGTTGGCATGGTAGC-3′ | 5′-CCCTGGACACCAACTATTGC-3′ |
| VEGF | 5′-CACACAGGATGGCTTGAAGA-3′ | 5′-AGGGCAGAATCATCACGAAG-3′ |
| IL-10 | 5′-TCAAACTCACTCATGGCTTTGT-3′ | 5′-GCTGTCATCGATTTCTTCCC-3′ |
| COX2 | 5′-GTTTTGACATGGGTGGGAAC-3′ | 5′-CCCTCAGACAGCAAAGCCTA-3′ |
| CD33 | 5′-TTCCTCCTGTGGGTCTTCAC-3′ | 5′-CTTTCCAGGAGATGGCTCAG-3′ |
| ETS1-96 | 5′-GATAGTTGTGATCGCCTCACC-3′ | 5′-GTCCTCTGAGTCGAAGCTGTC-3′ |
| ETS1-237 | 5′-TACACAGGCAGTGGACCAATC-3′ | 5′-CCCCGCTGTCTTGTGGATG-3′ |
| GAPDH | 5′-CACACAGGATGGCTTGAAGA-3′ | 5′-AGGGCAGAATCATCACGAAG-3′ |
Suppression assay
Fresh T cells isolated from PBMCs using anti-CD3 microbeads (Miltenyi Biotec) were labeled with carboxyfluorescein diacetate succinimidyl ester (5 μM; Sigma-Aldrich, St. Louis, MO) and seeded at 105 cells/well. CD33+ cells isolated from cytokine induction systems with or without DXM modulation were added at 1:8, 1:4, or 1:2 ratios. T cells were stimulated with anti-human CD3 (eBioscience, San Diego, CA), anti-human CD28 (Biolegend, San Diego, CA) antibodies and 50 U/mL recombinant human IL-2 (rhIL-2; R&D Systems). All cells were incubated in triplicate for 5 days and acquired for fluorescence-activated cell sorter analysis. For each run, controls included T cells cultured with or without stimulation, as well as T cells cultured with CD33+ cells from medium only. Data were analyzed using Flow Jo software.
Cytotoxicity assay
Cytotoxic T lymphocytes (CTLs) were isolated from PBMCs of patients with ITP, using CD8 magnetic microbeads (Miltenyi Biotec) and cocultured with in vitro-generated MDSCs for 3 days. MDSC-modulated CTLs (105/mL) and autologous platelets (106/mL) served as effector cells and target cells, respectively. After 4 hours of incubation at 37°C, early apoptosis of platelets was detected using a mitochondrial membrane potential assay kit (Beyotime Biotechnology, Shanghai, China), per manufacturer’s instructions. Spontaneous apoptosis was measured in tubes containing medium and platelets only.
Immunofluorescence
Samples were prepared and slides stained with primary anti-CD11b or anti-iNOS antibodies, followed by incubation with anti-CD33 and secondary antibodies, as described in supplemental Methods. Images were taken on an Olympus FV1000 confocal laser scanning microscope and analyzed using Zen 2009 Light Edition software and Image-Pro plus 6.0.
siRNA transfection
Transfection with Ets1 small interfering RNA (siRNA), NC siRNA, and FAM siRNA were performed as described in supplemental Methods. Cells were incubated for a total of 48 hours and functionally evaluated by reverse transcription-polymerase chain reaction and so on.
Generation of MDSCs from murine bone marrow
MDSCs were generated from bone marrow (BM) of WT C57BL/6 mice. Femurs and tibias were collected, and BM was flushed out with sterile phosphate-buffered saline. BM cells were counted and seeded at a density of 106 cells/mL in Dulbecco’s Modified Eagle Medium (Sigma-Aldrich) containing 10% fetal bovine serum (Gibco, Grand Island, NY). BM cells were cultured for 3 days in the presence of recombinant murine (rm) granulocyte–macrophage colony-stimulating factor, rmIL-6, rmG-CSF (10 ng/mL each; Peprotech, Rocky Hill, NJ), and DXM.
Animal model and cell treatment
Blood was drawn by retro-orbital bleeding. Platelets were harvested by centrifugation and adjusted to 109/mL for immunization. C57BL/6 CD61 KO mice were transfused weekly with 100 μL of 108 platelets from WT mice for 3 consecutive weeks. The antiplatelet immune CD61 KO mice were killed, and their spleens were removed and prepared into a splenocyte suspension.26 On the day of splenocyte transfer, the SCID mice were subjected to 180 cGy total body irradiation to inhibit recipient innate immune responses and enhance engraftment. Within 3 hours of irradiation, certain mice were injected intraperitoneally with 100 μL of the indicated splenocyte preparations (at 5 × 104/mL final) and/or 6 × 106 MDSCs. Platelet counts were recorded weekly, and blood was diluted 1/100 before counting. The SCID mice were killed 4 weeks after irradiation. Spleens were removed, from which mRNA was extracted and further evaluated for Ets1 expression, as described in supplemental Methods.
Statistical analysis
Results were expressed as mean ± standard deviation or median (range). The statistical significance of the differences observed among unmodulated, DXM-modulated, and control groups was determined by analysis of variance. Differences between pre- and posttreatment groups were determined by paired Student t test, and the difference between 2 independent groups was compared using the nonpaired t test, unless the data were not normally distributed, in which case the Wilcoxon matched-pairs test and Mann-Whitney test were used. P < .05 was considered statistically significant.
Results
MDSCs in patients with ITP and control patients
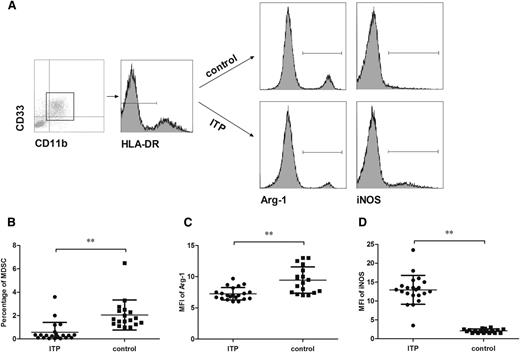
To evaluate the number of MDSCs in the peripheral blood of patients with ITP and healthy control patients, the surface expression of CD11b, CD33, and HLA-DR on circulating cells from whole blood was determined. A population of CD11b+CD33+HLA-DRlow cells was gated for analysis, and the number of MDSCs was calculated as a percentage of the gated cells within all live cells (Figure 1A). Results showed that the percentage of MDSCs in circulation was significantly lower in patients with ITP than in healthy control patients (P < .01; Figure 1B).
The number of MDSCs and their expression of Arg-1/iNOS in the peripheral blood. (A) The representative scatter-gram of CD11b+CD33+HLA-DRlow cells within the gate of PBMCs. Histograms of Arg-1 and iNOS in CD11b+CD33+HLA-DRlow cells from healthy control patients and patients with ITP before any treatment was initiated. (B) The percentage of CD11b+CD33+HLA-DRlow cells in hemolyzed whole blood from primary active patients with ITP (n = 21) and healthy control patients (n = 18). (C-D) The expression (mean fluorescence intensity) of Arg-1 (C) and iNOS (D) in circulating MDSCs compared between patients with ITP (n = 21) and control patients (n = 18). Significance between the 2 groups was determined by Student-Newman-Keuls test. Bars represent SD. **P < .01.
The number of MDSCs and their expression of Arg-1/iNOS in the peripheral blood. (A) The representative scatter-gram of CD11b+CD33+HLA-DRlow cells within the gate of PBMCs. Histograms of Arg-1 and iNOS in CD11b+CD33+HLA-DRlow cells from healthy control patients and patients with ITP before any treatment was initiated. (B) The percentage of CD11b+CD33+HLA-DRlow cells in hemolyzed whole blood from primary active patients with ITP (n = 21) and healthy control patients (n = 18). (C-D) The expression (mean fluorescence intensity) of Arg-1 (C) and iNOS (D) in circulating MDSCs compared between patients with ITP (n = 21) and control patients (n = 18). Significance between the 2 groups was determined by Student-Newman-Keuls test. Bars represent SD. **P < .01.
The cytoplasmic expression of Arg-1 and iNOS in circulating MDSCs was analyzed by flow cytometry after cell fixation and permeabilization. The expression level was represented as mean fluorescence intensity of Arg-1 and iNOS in CD11b+CD33+HLA-DRlow cells (Figure 1A). Significantly lower Arg-1 levels (median, 7.0 [range, 6.0-9.7] vs median, 9.3 [range, 7.0-13.0]) and higher iNOS levels (median, 13.0 [range, 3.5-23.5] vs median, 2.1 [range, 1.5-3.0]) were found in MDSCs of patients with ITP compared with healthy control patients (P < .01; Figure 1C-D).
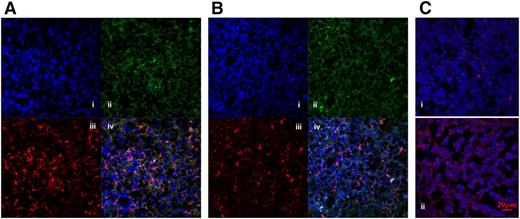
To locate splenic MDSCs of patients with ITP and non-ITP control patients, CD11b+CD33+ double-positive cells were detected using confocal laser scanning microscopy with a 40× objective lens. Images were taken as single plane per fluorescence and merged as series. CD11b+CD33+ double-positive cells were counted in each merged view. Our data demonstrated that the number of MDSCs in the spleen was greater in non-ITP control patients than in patients with ITP (5.8 ± 2.3 vs 2.6 ± 1.8; Figure 2A-B).
MDSCs in the spleens of control patients and patients with ITP. CD11b+CD33+ double-positive cells in spleen sections were visualized by immunofluorescence. Nuclei were counterstained with DAPI. Representative staining of (Ai, Bi) nuclei, (Aii, Bii) CD33, (Aiii, Biii) CD11b, and (Aiv, Biv) merged images of control (A) and ITP (B) spleens. CD11b+CD33+ cells were counted per merged view. The expression (mean fluorescence intensity; OD = IOD/area) of iNOS in splenic MDSCs from control patients (Ci) and patients with ITP (Cii). Original magnification, ×400 (spleen). Scale bars, 20 μm.
MDSCs in the spleens of control patients and patients with ITP. CD11b+CD33+ double-positive cells in spleen sections were visualized by immunofluorescence. Nuclei were counterstained with DAPI. Representative staining of (Ai, Bi) nuclei, (Aii, Bii) CD33, (Aiii, Biii) CD11b, and (Aiv, Biv) merged images of control (A) and ITP (B) spleens. CD11b+CD33+ cells were counted per merged view. The expression (mean fluorescence intensity; OD = IOD/area) of iNOS in splenic MDSCs from control patients (Ci) and patients with ITP (Cii). Original magnification, ×400 (spleen). Scale bars, 20 μm.
Intracellular staining with anti-iNOS antibody showed stronger fluorescence intensity (Image-Pro Plus 6.0) in slides of patients with ITP than non-ITP control patients, indicating that splenic MDSCs of patients with ITP had higher levels of iNOS than control patients on a semiquantitative scale (Figure 2C).
HD-DXM expands CD11b+CD33+HLA-DRlow cell populations
Alterations in the CD11b+CD33+HLA-DRlow cell population were monitored in patients with ITP before and after HD-DXM treatment. Data revealed that HD-DXM significantly elevated the percentage of circulating MDSCs in patients with ITP (P < .01; Figure 3A). To investigate the effect of DXM on MDSC expansion, changes in surface expression of CD33 and CD11b on cytokine-induced CD33+ cells from patients with ITP were also determined in vitro. After different concentrations of DXM (0, 0.02, 0.25, 0.5, 1, and 2 μM) were added, the percentages of CD11b+CD33+ cells in 8 randomly selected patients were evaluated. As shown in Figure 3B, DXM with concentration gradients from 0.02 to 2 μM could stimulate the expansion of MDSCs in a dose-dependent manner. We adopted an effective concentration of 0.5 μM based on the dose range and efficacy to confirm its modulatory effects on MDSCs in 8 additional patients. This concentration was within the range of plasma concentration that HD-DXM was administered in vivo.21 Together, the percentage of CD11b+CD33+ double-positive cells showed a significant increase after addition of DXM in the 16 patients compared with pretreatment baseline (62.77 ± 7.02 vs 84.02 ± 9.53; P < .01; Figure 3C). The data demonstrated that HD-DXM enhanced the population growth and promoted the proliferation of MDSCs in ITP.
HD-DXM promoted the expansion of MDSCs in patients with ITP. (A) Changes in the CD11b+CD33+HLA-DRlow cell population monitored before and after HD-DXM treatment (n = 21). (B) DXM with concentration gradients from 0.02 to 2 μM stimulated the expansion of MDSCs in a dose-dependent manner in 8 randomly selected patients in vitro. (C) Elevated percentages of CD11b+CD33+ cells after DXM (0.5 μM) modulation in vitro in cytokine-induced MDSCs from 16 patients with ITP. Differences between pre- and posttreatment groups were determined by paired Student t test. **P < .01.
HD-DXM promoted the expansion of MDSCs in patients with ITP. (A) Changes in the CD11b+CD33+HLA-DRlow cell population monitored before and after HD-DXM treatment (n = 21). (B) DXM with concentration gradients from 0.02 to 2 μM stimulated the expansion of MDSCs in a dose-dependent manner in 8 randomly selected patients in vitro. (C) Elevated percentages of CD11b+CD33+ cells after DXM (0.5 μM) modulation in vitro in cytokine-induced MDSCs from 16 patients with ITP. Differences between pre- and posttreatment groups were determined by paired Student t test. **P < .01.
Cytokine-induced CD33+ MDSCs have upregulated IL-10, transforming growth factor β, and vascular endothelial growth factor
The reported phenotype of human MDSCs is roughly CD33+HLA-DRlowCD11b+, with low expression of differentiated macrophage and DC markers.27,28 CD33+ cells from cytokine-treated cultures showed a significant increase in CD11b expression relative to isotype controls, with low to intermediate expression of antigen presentation protein HLA-DR. Thus, these cytokine-induced CD33+ cells phenotypically resembled human MDSCs.27
MDSC-mediated suppression on effector T-cell responses has been shown to correlate in part with increased expression of IL-10, iNOS, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF) β in suppressor cells.29,30 To better characterize the nature of in vitro-generated MDSCs, the above putative cytokine-driven mechanisms of MDSC function was evaluated by reverse-transcription polymerase chain reaction. Cytokine-induced MDSCs, both from PBMC and splenocytes of patients with ITP and healthy control patients, demonstrated significant upregulation of IL-10, TGF-β, and VEGF compared with noninduced systems. Furthermore, we found that DXM modulation significantly augmented the expression level of the above genes in cytokine-induced MDSCs. Therefore, DXM modulation proved to reinforce certain suppressive pathways involving IL-10, TGF-β, and VEGF levels in MDSCs (P < .05; Figure 4).
The upregulation of IL-10, TGF-β, and VEGF expression in cytokine-induced CD33+ cells. Expression levels of suppressive molecules pre- or postexposure to 0.5 μM DXM from patients with ITP (PBMC origin, n = 8; splenic origin, n = 5) and healthy control patients (PBMC origin n = 9; splenic origin n = 5). Each column denotes the relative mRNA quantity of IL-10, TGF-β, or VEGF in cytokine-induced CD33+ cells from (A-C) PBMCs or (D-F) splenocytes. Differences between PBMCs (ITP) and MDSCs (ITP), MDSCs (ITP) and DXM-MDSCs (ITP), or PBMCs (control) and MDSCs (control), MDSCs (control) and DXM-MDSCs (control) were determined using analysis of variance or the Wilcoxon matched-pairs test. *P < .05; **P < .01.
The upregulation of IL-10, TGF-β, and VEGF expression in cytokine-induced CD33+ cells. Expression levels of suppressive molecules pre- or postexposure to 0.5 μM DXM from patients with ITP (PBMC origin, n = 8; splenic origin, n = 5) and healthy control patients (PBMC origin n = 9; splenic origin n = 5). Each column denotes the relative mRNA quantity of IL-10, TGF-β, or VEGF in cytokine-induced CD33+ cells from (A-C) PBMCs or (D-F) splenocytes. Differences between PBMCs (ITP) and MDSCs (ITP), MDSCs (ITP) and DXM-MDSCs (ITP), or PBMCs (control) and MDSCs (control), MDSCs (control) and DXM-MDSCs (control) were determined using analysis of variance or the Wilcoxon matched-pairs test. *P < .05; **P < .01.
The effect of DXM treatment on suppressive functions of cytokine-induced MDSCs
The ability of cytokine-induced CD33+ cells to inhibit autologous T-cell proliferation was analyzed by flow cytometry. Results showed that the in vitro-generated CD33+ MDSCs were capable of suppressing CD4+ T-cell proliferation at 1:4 and 1:2 ratios by 41.5% and 77.9%, respectively. For each sample run, the number of peaks was counted and the division index was calculated. The proliferation of CD4+ T cells was significantly inhibited, whereas the addition of stimulus alone or CD33+ cells in medium only had no remarkable effect. Moreover, CD33+ MDSCs induced in the presence of DXM significantly enhanced the suppression of T-cell division at 1:4 and 1:2 ratios by 25% and 35%, respectively (P < .01; Figure 5A-C).
DXM modulation enhanced CD4+ and CD8+ T cell suppression by cytokine-induced CD33+ cells. (A) The inhibitory effect of in vitro-generated MDSCs on CD4+ T-cell proliferation. The number of peaks resembles cell division processes in DXM-modulated and unmodulated groups. (B-C) The division index of lymphocyte proliferation using in vitro-generated MDSCs from 8 randomly selected patients at 1:2 (B) and 1:4 (C) ratios. (D) A representative scatter-gram of JC1 mitochondrial potential test for platelet apoptosis. JC1 is a mitochondrial membrane potential-sensitive carbocyanine probe. Monomeric green fluorescent JC1 is taken up by high-membrane potential mitochondria, where it reversibly forms red fluorescent aggregates with loss of mitochondrial membrane potential. (E) Apoptosis level of platelets (PLT) alone (2.714 ± 0.296), platelets cocultured with CD8+ T cells (CTL; 16.094 ± 2.676), platelets cocultured with MDSC-primed CD8+ T cells (9.992 ± 1.431), and platelets cocultured with DXM-MDSC-primed CD8+ T cells (6.206 ± 0.981), using in vitro-generated MDSCs from 10 randomly selected patients. Differences between each 2 groups were compared using the Mann-Whitney test. **P < .01.
DXM modulation enhanced CD4+ and CD8+ T cell suppression by cytokine-induced CD33+ cells. (A) The inhibitory effect of in vitro-generated MDSCs on CD4+ T-cell proliferation. The number of peaks resembles cell division processes in DXM-modulated and unmodulated groups. (B-C) The division index of lymphocyte proliferation using in vitro-generated MDSCs from 8 randomly selected patients at 1:2 (B) and 1:4 (C) ratios. (D) A representative scatter-gram of JC1 mitochondrial potential test for platelet apoptosis. JC1 is a mitochondrial membrane potential-sensitive carbocyanine probe. Monomeric green fluorescent JC1 is taken up by high-membrane potential mitochondria, where it reversibly forms red fluorescent aggregates with loss of mitochondrial membrane potential. (E) Apoptosis level of platelets (PLT) alone (2.714 ± 0.296), platelets cocultured with CD8+ T cells (CTL; 16.094 ± 2.676), platelets cocultured with MDSC-primed CD8+ T cells (9.992 ± 1.431), and platelets cocultured with DXM-MDSC-primed CD8+ T cells (6.206 ± 0.981), using in vitro-generated MDSCs from 10 randomly selected patients. Differences between each 2 groups were compared using the Mann-Whitney test. **P < .01.
CTL-mediated platelets apoptosis was evaluated in patients with ITP after premodulation of CTL by cytokine-induced MDSCs in the presence or absence of DXM. As a result, CTLs induced significant autologous platelet apoptosis in patients with ITP. In these patients, MDSC-primed CTLs showed weaker cytotoxic effects and resulted in a significantly lower platelet apoptosis level compared with unprimed CTLs. The reduced cytotoxicity was further demonstrated in the presence of DXM with even fewer platelets undergoing apoptosis (P < .01; Figure 5D-E).
The association of Ets1 with DXM modulation
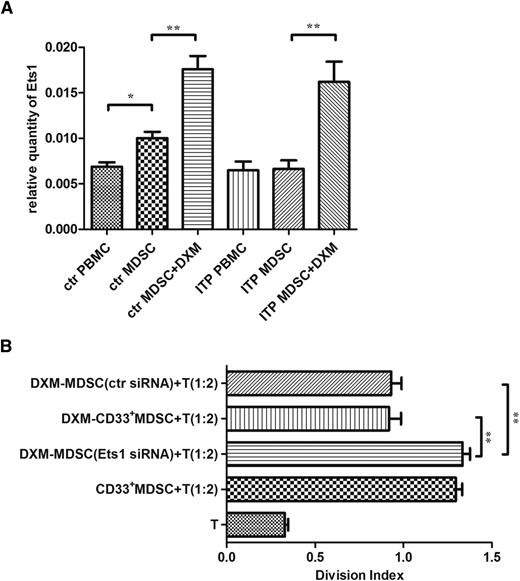
The relative quantitative mRNA level of Ets1 was significantly higher in DXM-modulated MDSCs than in nonmodulated cells, indicating that the transcription factor Ets1 might be associated with DXM modulation (P < .01; Figure 6A).
The contribution of Ets1 to DXM modulation. (A) Expression levels of Ets1 in MDSCs pre- or postexposure to 0.5 μM DXM from patients with ITP (n = 21) and healthy control patients (n = 18). (B) Changes in suppressive activity of DXM-modulated MDSCs toward CD4+ T-cell proliferation after Ets1 siRNA transfection (n = 8). The division index of lymphocytes cocultured with DXM-MDSCs or transfected DXM-MDSCs at a 2:1 ratio. Differences between each 2 groups were compared using the Mann-Whitney test. **P < .01.
The contribution of Ets1 to DXM modulation. (A) Expression levels of Ets1 in MDSCs pre- or postexposure to 0.5 μM DXM from patients with ITP (n = 21) and healthy control patients (n = 18). (B) Changes in suppressive activity of DXM-modulated MDSCs toward CD4+ T-cell proliferation after Ets1 siRNA transfection (n = 8). The division index of lymphocytes cocultured with DXM-MDSCs or transfected DXM-MDSCs at a 2:1 ratio. Differences between each 2 groups were compared using the Mann-Whitney test. **P < .01.
The siRNA transfection produced a successful silencing of the Ets1 gene, as confirmed in DXM-modulated MDSCs transfected with Ets1 siRNA, which resulted in significantly lower level of Ets1 mRNA compared with the NC siRNA or FAM siRNA transfections. The suppression of CD4+ autologous T-cell proliferation by DXM-modulated MDSCs after Ets1 siRNA transfection was significantly attenuated compared with that by mock-transfected MDSCs, which demonstrated an evidential function decline. The enhancement of MDSC function elicited by DXM modulation, as described previously, was eventually offset by excluding the effect of Ets1 (P < .01; Figure 6B).
MDSC treatment alleviated thrombocytopenia in a murine model of ITP
CD61+-SCID mice engrafted with 5 × 104 splenocytes from CD61 KO mice immunized against CD61+-platelets exhibited profound thrombocytopenia, and there was a proportion of bleeding mortality within 21 days after transfer. Bleeding diathesis occurred in the intestines, lungs, abdomen, subcutaneous tissues, and brain. The transferred SCID mice had significant levels of serum antiplatelet CD61-specific IgG antibodies to indicate model criteria by the second week.
The SCID mice were separated into 4 groups. Group 1 received an intravenous injection of 6 × 106 MDSCs generated from WT BM in addition to the splenocyte engraftment, which was administered on the day of and 2 days after the splenocyte transfer. Group 2 received an intravenous injection of the same volume of phosphate-buffered saline, such that splenocytes were the only cell treatment (negative control). Group 3 received the same amount of MDSCs as group 1 but did not receive splenocyte engraftment. Group 4 did not receive any cell transfer at all (blank control).
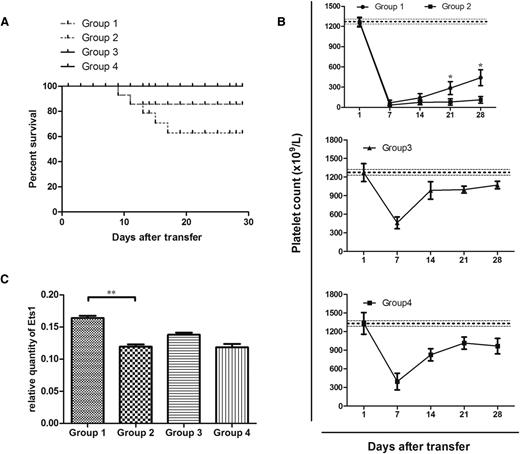
Group 1 demonstrated an overall higher survival rate than group 2. According to the routinely monitored platelet counts, mice in group 1 showed significantly higher platelet levels than mice in group 2 at the third and fourth weeks after irradiation. All mice in groups 3 and 4 recovered from irradiation-induced thrombocytopenia by day 14, and mice in group 3 showed no statistical fluctuation of platelet number compared with mice in group 4 (Figure 7A-B).
MDSC treatment alleviated thrombocytopenia in mice. (A) Kaplan-Meier survival plots of irradiated SCID mice transferred with (group 1) 5 × 104 immune splenocytes from CD61 KO mice immunized against WT platelets and 6 × 106 MDSCs generated from WT BM (n = 10), (group 2) 5 × 104 immune splenocytes (n = 15), (group 3) 6 × 106 MDSCs (n = 5), or (group 4) no cells at all (n = 5). The data are expressed as the percentage of survival. (B) Platelet counts in irradiated SCID mice from groups 1 to 4. Thrombocytopenia occurred in all mice on day 7. The data are expressed as platelet counts (×109/L) ± SEM over time (days). The horizontally dotted lines represent the normal platelet range. (C) Differences in gene expression of SCID splenic Ets1 in association with different cell treatments administered. Significance among groups was determined by Mann-Whitney test or analysis of variance. **P < .01.
MDSC treatment alleviated thrombocytopenia in mice. (A) Kaplan-Meier survival plots of irradiated SCID mice transferred with (group 1) 5 × 104 immune splenocytes from CD61 KO mice immunized against WT platelets and 6 × 106 MDSCs generated from WT BM (n = 10), (group 2) 5 × 104 immune splenocytes (n = 15), (group 3) 6 × 106 MDSCs (n = 5), or (group 4) no cells at all (n = 5). The data are expressed as the percentage of survival. (B) Platelet counts in irradiated SCID mice from groups 1 to 4. Thrombocytopenia occurred in all mice on day 7. The data are expressed as platelet counts (×109/L) ± SEM over time (days). The horizontally dotted lines represent the normal platelet range. (C) Differences in gene expression of SCID splenic Ets1 in association with different cell treatments administered. Significance among groups was determined by Mann-Whitney test or analysis of variance. **P < .01.
The differences among groups in splenic gene expression of mouse Ets1 was found to be associated with cell treatments administered to the SCID mice. Mice in group 1 had a significantly elevated level of Ets1 expression in comparison with mice in group 2. This indicated that Ets1 might be associated with the functioning of DXM-modulated MDSCs (P < .01; Figure 7C).
Discussion
Antiplatelet autoantibodies and T-cell-mediated cytotoxicity31 have accelerated platelet destruction and suppressed platelet production in ITP.32 Defective regulatory T cells33,34 and regulatory B cells35 were involved in the loss of immune tolerance in ITP. Identified as a contributor to immune regulation, MDSCs possess an arsenal of immunosuppressive properties mediated in part by the production of TGF-β and IL-10.36 MDSCs consist of 2 major subsets: granulocytic and monocytic MDSCs. The granulocytic subset produces little nitric oxide (NO), whereas the monocytic subset has upregulated expression of iNOS, and thus increased levels of reactive nitrogen oxide species. Both subsets have an elevated level of Arg-1 activity. Nitric oxide and Arg-1 restrain exuberant or novel T-cell responses via a variety of different mechanisms,37-39 by interfering with direct TLR signaling and MyD88-dependent activation of nuclear factor-кB (NF-кB).40-42
In this study, the percentages of both circulating and splenic MDSCs were reduced in patients with ITP compared with healthy control patients. In the peripheral blood, the lower Arg-1 level in manifested with patients with ITP subdued enzymatic reaction, and thus retarded suppressive function of MDSCs compared with that of control patients; in contrast, the higher iNOS level in patients with ITP represented an aberrance in the l-arginine metabolic systems of MDSCs. Consistently, in peripheral lymphoid organs, splenic MDSCs of patients with ITP with positive iNOS expression outnumbered those of healthy control patients, indicating altered functional characteristics in T-cell suppression.
As shown in supplemental Table 1, HD-DXM led to ideal initial responses20,21 in previously untreated active adult patients. And clinical response to steroid therapy was associated with MDSC improvement in patients with ITP (supplemental Figure 1). HD-DXM could resume the disturbed Th1/Th2 balance and shift their cytokine production back to normal,43,44 establishing a primal molecular environment to restore impaired cell populations, such as MDSCs. In the present study, HD-DXM successfully increased the number of MDSCs in patients with ITP, which was further demonstrated by an augmentation of in vitro-generated patient MDSCs. More intriguingly, DXM was capable of resuming the functioning of various immunosuppressive processes in MDSCs, including but not limited to the production of suppressive cytokines and inhibition of both CD4+ and CD8+ T lymphocytes.45 The expansion of TGF-β and IL-10 mRNA in DXM-modulated MDSCs implicated a novel role for glucocorticoids in immunosuppression. After DXM modulation, VEGF was also upregulated significantly in cytokine-induced MDSCs of PBMC origin, but not as much in MDSCs of splenic origin, probably because of its relatively high baseline expression in the spleen. MDSCs generated in vitro did not show significant differences in the above mRNA profile between patients with ITP and healthy control patients, suggesting that samples from patients with ITP possess the same potency in MDSC induction. This potential laid the foundation of glucocorticoid efficacy toward MDSCs. Enhanced control over CD4+ T-cell proliferation and CTL-mediated platelet lysis further established the role of glucocorticoids in reconstructing MDSC function. However, our data indicated that DXM did not expand the T regulatory cell Tr1 through MDSCs, and DXM-MDSCs might exert their suppressive effects through mechanisms other than the induction of Tr1 in ITP (supplemental Figure 2). Although cytotoxicity-driven platelet destruction is only regarded as a minor cause of thrombocytopenia in ITP, it remains an important mechanism that accounts for autoantibody-negative scenarios. Li et al previously reported that in patients with chronic ITP, activated CD8+ T cells in bone marrow might suppress megakaryocyte maturation/apoptosis, leading to impaired platelet production, which could be successfully corrected by DXM.46 And more recently, Ma et al uncovered in murine ITP a subset of CD8+ T regulatory cells with immunosuppressive potency expanded by steroid therapy.47 However, this did not appear to be the case in our human experiment, where robust cytotoxicity is detected and acted on by other suppressive cells such as MDSCs. Hence, attenuated cytotoxicity toward platelets confirmed the improvement of MDSC function by DXM.
The transcription factor Ets-1 is required for the development of natural Tregs.48 Ets-1 deficiency leads to altered B-cell differentiation, hyper-responsiveness to TLR9, and increased IL-17 producing T-helper cells, which eventually contribute to autoimmune diseases.49 Studies have indicated reduced expression of Ets1 in PBMCs of patients with systemic lupus erythematosus and patients with inflammatory bowel disease.50,51 Ets1 has also been reported to regulate the suppressive activity of MDSCs in tolerance induction to CD8+ T cells.52 Glucocorticoids modulate cell fate by altering gene expression via the glucocorticoid receptor (GR). GRs can interact directly or indirectly with other transcription factors such as Ets1.53 Having a direct crosstalk with GR, during which the GR and other transcription factors modulate each other's activity by binding to the promoters of certain target genes, Ets1 can act as a molecular switch to mediate steroid actions.53,54 Upon silencing Ets1 in the DXM-modulated MDSCs, the suppression of CD4+ T-cell proliferation was significantly quenched. The results indicated a possible pathway through which DXM was capable of correcting the malfunction of MDSCs in ITP.
Finally, in vivo outcomes of adoptive cell transfer with MDSCs was evaluated in mice. Our animal experiments elucidated that MDSC treatment alleviated thrombocytopenia and resulted in a higher survival rate in the ITP murine model. Mice transferred with MDSCs in addition to the splenocyte engraftment had significantly higher expression of Ets1 than mice injected with splenocytes alone, which corresponded with the transfection data that Ets1 might be associated with the functioning of DXM-modulated MDSCs. In addition to consolidating the effect of DXM-modulated MDSCs, the experiment provided a therapeutic application of MDSCs via in vitro expansion followed by cell transplantation.
In summary, impaired MDSCs are involved in the immunopathogenesis of ITP. HD-DXM promoted the expansion and enhanced the suppressive function of MDSCs in patients with ITP. The effect of DXM-modulation on MDSCs was authenticated to correlate with the transcription factor Ets1. Our study sheds new light on immune therapeutic avenues targeting MDSCs in the management of ITP and suggests an innovative mechanism underlying the action of traditional glucocorticoids.
Presented at the 25th Congress of the International Society on Thrombosis and Haemostasis, Toronto, Ontario, Canada, June 20-25, 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Major Research plan of the National Natural Science Foundation of China (91442204), National Natural Science Foundation for Distinguished Young Scholars of China (81125002), National Natural Science Foundation of China (81270578, 81370623, and 81470284), State Program of National Natural Science Foundation of China for Innovative Research Group (81321061), State Key Clinical Specialty of China for Blood Disorders, and Tai Shan Scholar Foundation.
Authorship
Contribution: Y.H. and J.P. designed and performed research, analyzed data, and wrote the paper; Q.F., M.X., G.-s.L., X.-n.L., Z.S., Y.-x.S., and Y.-y.Y. performed research and analyzed data; H.Z., J.M., Y.W., J.-h.Q., and L.-l.S. evaluated the data and corrected the paper; X.-g.L. and M.H. designed and performed research and reviewed the work; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, 107 Wenhuaxi Rd, Jinan 250012, China; e-mail: junpeng88@sina.com.cn; and Ming Hou, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Jinan 250012, China; e-mail: qlhouming@sina.com.cn.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal