Key Points
Pomalidomide selectively targets BCL11A and SOX6 to induce γ-globin synthesis.
The mechanism of action of pomalidomide during erythropoiesis is independent of IKZF1 degradation, in contrast to multiple myeloma.
Abstract
Current therapeutic strategies for sickle cell anemia are aimed at reactivating fetal hemoglobin. Pomalidomide, a third-generation immunomodulatory drug, was proposed to induce fetal hemoglobin production by an unknown mechanism. Here, we report that pomalidomide induced a fetal-like erythroid differentiation program, leading to a reversion of γ-globin silencing in adult human erythroblasts. Pomalidomide acted early by transiently delaying erythropoiesis at the burst-forming unit-erythroid/colony-forming unit-erythroid transition, but without affecting terminal differentiation. Further, the transcription networks involved in γ-globin repression were selectively and differentially affected by pomalidomide including BCL11A, SOX6, IKZF1, KLF1, and LSD1. IKAROS (IKZF1), a known target of pomalidomide, was degraded by the proteasome, but was not the key effector of this program, because genetic ablation of IKZF1 did not phenocopy pomalidomide treatment. Notably, the pomalidomide-induced reprogramming was conserved in hematopoietic progenitors from individuals with sickle cell anemia. Moreover, multiple myeloma patients treated with pomalidomide demonstrated increased in vivo γ-globin levels in their erythrocytes. Together, these data reveal the molecular mechanisms by which pomalidomide reactivates fetal hemoglobin, reinforcing its potential as a treatment for patients with β-hemoglobinopathies.
Introduction
Under steady-state conditions, a healthy individual produces about 2 million erythrocytes every second. Erythropoiesis and red blood cell (RBC) turnover are tightly regulated, ensuring maintenance of constant levels of hemoglobin in the circulation. Increased red cell destruction and/or decreased production lead to a decrease in hemoglobin concentration and anemia, a major cause of morbidity worldwide.1
β-hemoglobinopathies, such as β-thalassemia and sickle cell disease (SCD), represent a subclass of anemia caused by mutations in the HBB gene, encoding the β-globin chain. Because of their prevalence and severity, they impose a serious health care burden, particularly in the developing world.2 Therefore, β-hemoglobinopathies have been the focus of numerous studies aimed at elucidating the molecular pathophysiology of these diseases and developing new therapeutic strategies.3
Although the pathophysiology of β-thalassemia and SCD differ,4-9 clinical symptoms do not manifest before birth because β-globin chains are expressed postnatally. Because reactivation of fetal hemoglobin (HbF) ameliorates anemia and associated complications of SCD,10 strategies for HbF induction have long been pursued as therapeutic options.
Hydroxyurea (HU) is the only Food and Drug Administration (FDA)-approved drug for stimulating HbF in SCD patients.11 Although inexpensive and well-tolerated, the salutary effect of HU is limited and development of new therapies is still needed.12 Because of the predominance of SCD in the developing world, emerging gene therapy strategies and hematopoietic stem cell transplantation, although appealing,13 are likely to be of limited use in these countries because of limited technical and financial resources.14
Pomalidomide (CC-4047) is an FDA-approved third-generation immunomodulatory drug, originally developed to have increased antimyeloma activity as well as reduced side effects compared with thalidomide.15-17 In multiple myeloma (MM) cells, pomalidomide binds to cereblon and activates its E3-ligase activity, leading to ubiquitination and proteasomal degradation of Ikaros (IKZF1) and Aiolos (IKZF3), resulting in myeloma cytotoxicity.18,19
Importantly, pomalidomide appears to have significant effects on human erythropoiesis. Although controversial, early in vitro studies on pomalidomide suggested that it induces a shift in lineage commitment by suppressing erythropoiesis while promoting myelopoiesis.20 However, further studies demonstrated that pomalidomide induces HbF production by promoting erythropoiesis both in vitro and in vivo, using a humanized mouse model of SCD.21,22 Nevertheless, the molecular pathway by which pomalidomide modulates erythropoiesis, leading to HbF induction, is unknown. Using a modified 3-phase liquid culture system that recapitulates human erythropoiesis23 to differentiate control and SCD-derived CD34+ hematopoietic stem and progenitor cells (HSPCs), we present evidence that pomalidomide reverses γ-globin silencing through transcriptional reprogramming of erythroid progenitors.
Material and methods
CD34+ cell isolation, expansion, and three-phase culture system
All studies involving human samples were conducted in accordance with the declaration of Helsinki and under institutional review board (IRB) approval of the North Shore-LIJ Health System. CD34+ cells were isolated from deidentified cord blood, deidentified control patient peripheral blood leukoreduction filters, or 50 mL of phlebotomized sickle cell patient peripheral blood. Sickle cell patients were enrolled and consented according to the North Shore-LIJ Health System IRB approval. To limit variability from one individual to another, CD34+ cells isolated from 20 leukoreduction filters were pooled for each experiment. Blood components were separated using Ficoll-Opaque, and CD34+ cells were purified using anti-CD34 conjugated microbeads and manual cell separation columns as per the manufacturer’s instructions (Miltenyi). Isolated peripheral blood CD34+ cells were expanded in H3000 media supplemented with CC100 (Stem Cell Technologies) for 4 days at a density of 105 cells/mL. CD34+ cells were differentiated toward erythrocytes using an adapted version of a previously described in vitro three-phase culture system.24 Briefly, CD34+ cells were cultured in 3% (vol/vol) albumin serum, 2% (vol/vol) human plasma, 10 μg/mL insulin, 3 U/mL heparin, 200 μg/mL transferrin supplemented with either 10 ng/mL stem cell factor, 1 ng/mL interleukin-3 (IL-3), and 3 IU/mL erythropoietin (EPO) (phase 1; D0-7) or 10 ng/mL SCF and 3 IU/mL EPO (phase 2; D7-11) or 1 mg/mL transferrin and 3 IU/mL EPO (phase 3; D11-16). 1 μM pomalidomide, 10 μM HU, or dimethyl sulfoxide (DMSO) was added to erythroid differentiation cultures where indicated. Pomalidomide dosing for in vitro experiments was based on a previously published report examining the maximal induction of HbF while limiting cell toxicity21 and our own data (supplemental Figure 1, available on the Blood Web site.).
Multiple myeloma patient samples
Deidentified freshly phlebotomized blood from patients with MM treated with pomalidomide was obtained in accordance with North Shore-LIJ Health System IRB approval. The only information obtained from these patients was the dose of pomalidomide and duration of treatment at the time of phlebotomy. Three patient samples were obtained, and 2 different time points were assayed to assess γ-globin induction. Patient #1 was treated with 4 mg pomalidomide for 3 weeks (first time point: 3 weeks), and then their dose was reduced to 2 mg for 9 weeks (second time point: 3 months). Patient #2 was treated with 4 mg pomalidomide for 9 months (first time point: 6 months; second time point: 9 months). Patient #3 was dosed with 2 mg pomalidomide for 2 months (first time point: 3 weeks; second time point: 2 months).
Flow cytometric analysis of erythropoiesis
Erythroid precursors and erythroblast maturation were assessed using 2 sets of cell surface markers as previously described.24,25 Burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E) populations were quantified on day 2 and day 4 of differentiation, and 105 cells were stained with a cocktail of antibodies consisting of an anti-IL-3R PE-Cy7–conjugated, anti-glycophorin A (GPA) PE–conjugated, anti-CD34 fluorescein isothiocyanate–conjugated and anti-CD36 allophycocyanin-conjugated for 15 minutes at room temperature. IL-3Rneg/GPAneg cells were gated on, and the expression of CD36 and CD34 was measured in double-negative cells. CD34hi/CD36lo and CD34lo/CD36hi populations characterize BFU-E and CFU-E, respectively.
Terminal erythroblast differentiation was monitored at days 7, 11, 14, and 16 of culture. 105 cells were pelleted and stained with a cocktail of antibodies consisting of an anti-band 3 fluorescein isothiocyanate–conjugated (produced by Dr Mohandas Narla), anti-GPA PE–conjugated, and anti-α4-integrin allophycocyanin–conjugated (MACS) antibodies for 15 minutes at room temperature. Erythroblasts were defined as GPApos cells, and the level of maturation was determined by α4 and band 3 expression. Immature erythroblasts (ie, proerythroblasts) were α4-integrinhi/band 3lo, whereas more differentiated erythroblasts such as orthochromatic and reticulocytes represent α4-integrinlo/band 3hi populations. Dead cells were excluded from analysis through 7-aminoactinomycin D staining. Flow cytometric analyses were performed on a BD Fortessa cytometer and FlowJo software. Unless otherwise indicated, all antibodies used were from BD Biosciences.
Lentivirus, transduction, and IKZF1 knockdown culture
IKZF1 knockdown studies were carried out using 2 different lentivirally encoded shRNA constructs against IKZF1 derived from IKZF1 MISSION shRNA Bacterial Glycerol Stock (Clone ID: NM_006060.3-1710s21c1 and NM_006060.3-3884s21c1; Sigma Aldrich). Lentivirally encoded shRNA to luciferase was used as a control. Information regarding transduction, selection, and differentiation can be found in the supplemental Methods, available on the Blood Web site.
Statistics
The data presented from control peripheral blood-derived CD34+ cells represent the average of independently performed biological replicates (N) containing pooled samples of 20 individuals. SCD CD34+ cells experiments reflect the average of independent experiments (N) using 7 different patient samples; these samples were not pooled. All statistical analyses were calculated using an unpaired 2-tailed Student t test. P < .05 was considered statistically significant. Details regarding exact P values and biological replicates are described within the figure legends.
Other methods
Detailed explanations of western blot, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), high-performance liquid chromatography (HPLC), proteasome inhibitor studies, cytoprep staining and flow cytometric analysis of F-cells, cell cycle, and apoptosis protocols are described in the supplemental Methods section.
Results
Pomalidomide reverses γ-globin silencing during adult human erythropoiesis in vitro
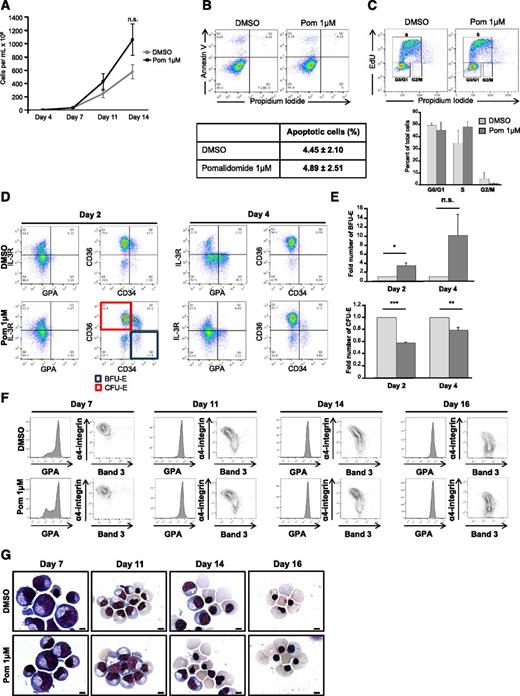
Pomalidomide induces HbF in vitro21 and in vivo using a humanized mouse model of SCD.22 However, whether increases in HbF by pomalidomide reflect a bona fide hemoglobin switch remains uncertain. To investigate this, we isolated and expanded adult peripheral blood CD34+ hematopoietic stem and progenitor cells, and differentiated them in the presence of 1 μM pomalidomide (previously determined21 and supplemental Figure 1A-D) using a 3-phase culture system that recapitulates human erythropoiesis including enucleation (Figure 1A).24 After 14 days of culture, pomalidomide induced reciprocal globin gene expression, with increased γ-globin and concomitant decreased β-globin expression (Figure 1B-C). Levels of α-globin chain were unaffected (Figure 1C).
Pomalidomide partially reverses the fetal-to-adult globin switch through the reciprocal expression of globin genes. (A) Schematic representation of human erythropoiesis and experimental design. Adult CD34+ cells from peripheral blood were expanded for 4 days, then differentiated using a three-phase culture system that recapitulates human erythropoiesis up to the enucleated reticulocyte (phases 1 to 3, detailed in the table at the bottom). 1 μM pomalidomide (Pom 1μM) was added at the end of the expansion phase and replaced every 3 to 4 days for the duration of the culture unless otherwise stated. (B) qRT-PCR of γ- and β-globin mRNA levels normalized to β-actin at D11 of differentiation in the presence of Pom 1μM or DMSO control. The data are shown as the mean fold difference of DMSO ± standard error of the mean (SEM) (N = 5): HBB (***P = 8.0785 × 10−5), HBG1 (**P = .0034), HBG2 (n.s. P = .0803). (C-I) D14 of differentiation: (C) western blot of cell lysates for α-, β-, and γ-globin chains (N = 3), and (D) hemoglobin production was assessed by HPLC using supernatants from dH2O lysed cells. Representative chromatograms from DMSO and pomalidomide-treated cultures. Gray and white arrows denote fetal hemoglobin (HbF) and adult hemoglobin (HbA), respectively. (E-F) The area under the curve was calculated, and relative hemoglobin composition was expressed as (E) the mean percent HbF/(HbF + HbA) ± SEM (N = 6): ***P = 5.5585 × 10−7 and (F) the mean percent HbA/(HbA + HbF) ± SEM (N = 6): ***P = 6.2138 × 10−7. (G) Cells were fixed, permeabilized, and stained with a PE-conjugated anti-HbF antibody. Flow cytometry data displayed as HbF against FSC. Box delineates HbFpos cells (F-cells), which possess a fluorescent intensity greater than unstained control (N = 3). (H) Western blot of γ-globin chain expression and (I) F-cells in cultures treated with Pom 1μM for 14 days, the first or second 7 days of differentiation (N = 3).
Pomalidomide partially reverses the fetal-to-adult globin switch through the reciprocal expression of globin genes. (A) Schematic representation of human erythropoiesis and experimental design. Adult CD34+ cells from peripheral blood were expanded for 4 days, then differentiated using a three-phase culture system that recapitulates human erythropoiesis up to the enucleated reticulocyte (phases 1 to 3, detailed in the table at the bottom). 1 μM pomalidomide (Pom 1μM) was added at the end of the expansion phase and replaced every 3 to 4 days for the duration of the culture unless otherwise stated. (B) qRT-PCR of γ- and β-globin mRNA levels normalized to β-actin at D11 of differentiation in the presence of Pom 1μM or DMSO control. The data are shown as the mean fold difference of DMSO ± standard error of the mean (SEM) (N = 5): HBB (***P = 8.0785 × 10−5), HBG1 (**P = .0034), HBG2 (n.s. P = .0803). (C-I) D14 of differentiation: (C) western blot of cell lysates for α-, β-, and γ-globin chains (N = 3), and (D) hemoglobin production was assessed by HPLC using supernatants from dH2O lysed cells. Representative chromatograms from DMSO and pomalidomide-treated cultures. Gray and white arrows denote fetal hemoglobin (HbF) and adult hemoglobin (HbA), respectively. (E-F) The area under the curve was calculated, and relative hemoglobin composition was expressed as (E) the mean percent HbF/(HbF + HbA) ± SEM (N = 6): ***P = 5.5585 × 10−7 and (F) the mean percent HbA/(HbA + HbF) ± SEM (N = 6): ***P = 6.2138 × 10−7. (G) Cells were fixed, permeabilized, and stained with a PE-conjugated anti-HbF antibody. Flow cytometry data displayed as HbF against FSC. Box delineates HbFpos cells (F-cells), which possess a fluorescent intensity greater than unstained control (N = 3). (H) Western blot of γ-globin chain expression and (I) F-cells in cultures treated with Pom 1μM for 14 days, the first or second 7 days of differentiation (N = 3).
Using HPLC to assess the quantity of HbF at day 14, pomalidomide-treated cultures demonstrated five- to sixfold elevation in HbF levels, whereas treatment with HU increased HbF only twofold (Figure 1D-E and supplemental Figure 1A). Moreover, pomalidomide attenuated HbA by 25% compared with vehicle control (Figure 1F). This was also observed to a lesser extent in HU-treated cultures (supplemental Figure 1B). Measurements of F-cells (ie, RBCs containing a significant amount of HbF) by flow cytometry in both pomalidomide and HU induced a pancellular distribution of HbF; yet, pomalidomide-treated cultures contained a higher percentage of F-cells (Figure 1G and supplemental Figure 1C). Pomalidomide appeared to act early during erythropoiesis as treatment from days 0-7, but not days 7-14, produced similar levels of γ-globin and F-cells when compared with cells exposed to pomalidomide during the entire culture period (Figure 1H-I).
Pomalidomide delays the maturation of early erythroid precursors without impairing terminal differentiation
Erythropoiesis was characterized in the absence or presence of pomalidomide. Cells exhibited comparable proliferation curves during the first 7 days of culture (Figure 2A). However, we noted a trend toward increased proliferation for cells treated with pomalidomide, from days 7-14, compared with control. Annexin-V, propidium iodide, and 5-ethynyl-2′-deoxyuridine staining at day 4 revealed that pomalidomide did not induce apoptosis or block cell cycle progression (Figure 2B-C).
Pomalidomide delays early erythropoiesis at the BFU-E/CFU-E transition, without altering cell proliferation, viability, and terminal erythroblast differentiation. (A) Growth curves of DMSO and pomalidomide-treated cultures. The data are shown as the mean cells/mL ± SEM (N = 6). (B-C) At day 4 of differentiation, cells were stained with (B) Annexin V and propidium iodide to assess apoptosis (N = 3) or (C) EdU and propidium iodide for cell cycle analyses (N = 3), along with the respective quantifications ± SEM. (D-E) Flow cytometric characterization of erythroid precursors and terminal erythroblast differentiation. (D) Using the surface markers IL-3R, GPA, CD36, and CD34, double-negative (IL-3Rneg/GPAneg) cells were gated and then analyzed for the expression of CD36 and CD34 to identify BFU-E (CD36neg/CD34hi) and CFU-E (CD36hi/CD34neg) populations. (E) Quantification of BFU-E and CFU-E at day 2 (BFU-E *P = .0202; CFU-E ***P = 1.4528 × 10−5) and day 4 (BFU-E n.s. P = .1272; CFU-E **P = .0088) represented as a fold of DMSO ± SEM (N = 3). (F) Terminal differentiation was monitored via α4-integrin and band3 levels of GPApos cells at indicated days. α4-integrinhi/band3lo cells represent less mature erythroblasts, whereas α4-integrinlo/band3hi cells are further differentiated (N = 6). (G) Representative May-Grunwald Giemsa–stained cytospins showing erythroblast morphology during terminal differentiation in the presence of Pom 1μM or DMSO control (N = 6). The bar represents 5 μm.
Pomalidomide delays early erythropoiesis at the BFU-E/CFU-E transition, without altering cell proliferation, viability, and terminal erythroblast differentiation. (A) Growth curves of DMSO and pomalidomide-treated cultures. The data are shown as the mean cells/mL ± SEM (N = 6). (B-C) At day 4 of differentiation, cells were stained with (B) Annexin V and propidium iodide to assess apoptosis (N = 3) or (C) EdU and propidium iodide for cell cycle analyses (N = 3), along with the respective quantifications ± SEM. (D-E) Flow cytometric characterization of erythroid precursors and terminal erythroblast differentiation. (D) Using the surface markers IL-3R, GPA, CD36, and CD34, double-negative (IL-3Rneg/GPAneg) cells were gated and then analyzed for the expression of CD36 and CD34 to identify BFU-E (CD36neg/CD34hi) and CFU-E (CD36hi/CD34neg) populations. (E) Quantification of BFU-E and CFU-E at day 2 (BFU-E *P = .0202; CFU-E ***P = 1.4528 × 10−5) and day 4 (BFU-E n.s. P = .1272; CFU-E **P = .0088) represented as a fold of DMSO ± SEM (N = 3). (F) Terminal differentiation was monitored via α4-integrin and band3 levels of GPApos cells at indicated days. α4-integrinhi/band3lo cells represent less mature erythroblasts, whereas α4-integrinlo/band3hi cells are further differentiated (N = 6). (G) Representative May-Grunwald Giemsa–stained cytospins showing erythroblast morphology during terminal differentiation in the presence of Pom 1μM or DMSO control (N = 6). The bar represents 5 μm.
BFU-E and CFU-E were estimated using flow cytometry (IL-3R, GPA, CD36, and CD34 as cell surface markers; Figure 2D and supplemental Figure 2A-D).25 The progression from BFU-E to CFU-E was affected by pomalidomide, with increased BFU-E and decreased CFU-E observed at both day 2 and day 4 (Figure 2D-E). Moreover, although CD34 expression was lost in controls, CD34 levels remained high in pomalidomide-treated cells (supplemental Figure 2A), suggesting that these cells retained their multilineage differentiation potential. Conversely, GPA expression was diminished at day 4, an effect that was sustained until day 7 (supplemental Figure 2B,F), suggesting that erythroblast commitment was delayed by pomalidomide. Monitoring erythroblast differentiation by GPA, α4-integrin, and band 324 did not show any defect in the terminal differentiation of GPApos cells (Figure 2F). Moreover, larger erythroblasts with normal morphology and macrocytic reticulocytes were observed (Figure 2G).
Pomalidomide differentially affects the main regulators of γ- and β-globin synthesis
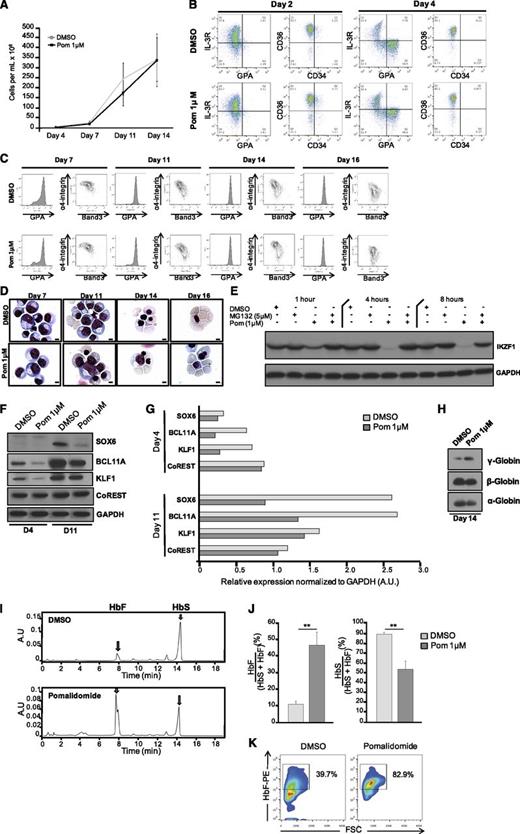
Extensive studies of globin switching have identified transcription factors and other regulators that form complex networks. Among these, BCL11A is recognized as a master regulator of γ-globin repression,3 but other studies have also implicated SOX6, IKZF1 (Ikaros), KLF1, LSD1, RCOR-1 (CoREST), MI2B, and GATA-1 in the regulation of the fetal-to-adult globin switch.26-30 qRT-PCR analyses revealed that BCL11A, SOX6, GATA1, KLF1, and LSD1 were downregulated by pomalidomide, whereas levels of IKZF1, MI2B, GATA2, HDAC1, and CoREST transcripts were unaffected at day 4 of differentiation (Figure 3A). By examining the protein levels at day 4 and day 11 of culture, we found that these proteins could be categorized into 3 different groups: those for which pomalidomide decreased their expression at first (day 4) and maintained decreased expression during differentiation (IKZF1, BCL11A, and SOX6; Figure 3B); those that were decreased at day 4 but normalized at day 11 (KLF1, GATA1, and LSD1; Figure 3C); and those that were not affected by pomalidomide (GATA2, CoREST, HDAC1; Figure 3D). These data demonstrate that pomalidomide exerted a transcriptional regulation of BCL11A and SOX6, as well as post-transcriptional regulation of IKZF1. In addition, quantification of the western blots demonstrated that the effect of pomalidomide was variable depending on the protein affected (Figure 3E), with the most significant and sustained effects observed with BCL11A, SOX6, and IKZF1 (>70% of reduction). The decrease in the expression levels of SOX6 was greatest at day 11, presumably because SOX6 is expressed later (compared with BCL11A) during erythroid differentiation.31
Pomalidomide treatment leads to selective targeting of the transcription networks modulating erythropoiesis and globin switching. (A) qRT-PCR of the main regulators of globin synthesis. The data are shown as the mean fold difference of DMSO ± SEM (N = 5): BCL11A (***P = .0004), SOX6 (**P = .0141), GATA1 (**P = .0113), KLF1 (**P = .0142), LSD1 (**P = .0051), IKZF1 (n.s. P = .1832), MI2B (n.s. P = .7901), GATA2 (n.s. P = .7667), HDAC1 (n.s. P = .1363), and CoREST (n.s. P = .1125). (B-D) Protein lysates from DMSO and pomalidomide-treated cells were collected at day 4 and day 11 of differentiation. The main transcription and chromatin remodeling factors involved in globin switching were investigated by immunoblot assays and grouped based on their expression patterns after pomalidomide treatment (N = 3): (B) IKZF1 (Ikaros), BCL11A, and SOX6 have reduced levels throughout the duration of culture. (C) GATA1, KLF1, and LSD1 display decreased expression early, but then normalize as differentiation progresses. (D) GATA2, CoREST, and HDAC1 are unaffected by pomalidomide. (E) Quantification of immunoreactive bands normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (F) Western blot for IKZF1 in cells that were dosed with DMSO, Pom 1μM, MG132 5μM, or a combination of Pom 1μM and MG132 5μM for 1, 4, and 8 hours. (G) Cultures were treated with DMSO, Pom 1μM, epoxomicin 1μM, or a combination of Pom 1μM and epoxomicin 1μM for 8 hours, and then immunoblotted for IKZF1. (H) At day 2 and day 4 of differentiation, cells were dosed with indicated treatments for 4 hours. Western blot of BCL11A and IKZF1.
Pomalidomide treatment leads to selective targeting of the transcription networks modulating erythropoiesis and globin switching. (A) qRT-PCR of the main regulators of globin synthesis. The data are shown as the mean fold difference of DMSO ± SEM (N = 5): BCL11A (***P = .0004), SOX6 (**P = .0141), GATA1 (**P = .0113), KLF1 (**P = .0142), LSD1 (**P = .0051), IKZF1 (n.s. P = .1832), MI2B (n.s. P = .7901), GATA2 (n.s. P = .7667), HDAC1 (n.s. P = .1363), and CoREST (n.s. P = .1125). (B-D) Protein lysates from DMSO and pomalidomide-treated cells were collected at day 4 and day 11 of differentiation. The main transcription and chromatin remodeling factors involved in globin switching were investigated by immunoblot assays and grouped based on their expression patterns after pomalidomide treatment (N = 3): (B) IKZF1 (Ikaros), BCL11A, and SOX6 have reduced levels throughout the duration of culture. (C) GATA1, KLF1, and LSD1 display decreased expression early, but then normalize as differentiation progresses. (D) GATA2, CoREST, and HDAC1 are unaffected by pomalidomide. (E) Quantification of immunoreactive bands normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (F) Western blot for IKZF1 in cells that were dosed with DMSO, Pom 1μM, MG132 5μM, or a combination of Pom 1μM and MG132 5μM for 1, 4, and 8 hours. (G) Cultures were treated with DMSO, Pom 1μM, epoxomicin 1μM, or a combination of Pom 1μM and epoxomicin 1μM for 8 hours, and then immunoblotted for IKZF1. (H) At day 2 and day 4 of differentiation, cells were dosed with indicated treatments for 4 hours. Western blot of BCL11A and IKZF1.
Pomalidomide targets Ikaros but not BCL11A for proteasomal degradation during erythroid differentiation
In MM cells, pomalidomide binds to cereblon and targets Ikaros (IKZF1) and Aiolos (IKZF3) for proteasomal degradation.18,19 Only IKZF1 is expressed during human erythropoiesis (supplemental Figure 3). Having found that IKZF1 levels were decreased only at the protein level in pomalidomide-treated erythroid cultures, we hypothesized that a similar mechanism functioned in the erythroid system. Pomalidomide-treated cells were cultured in the presence of 5 μM MG132 (Figure 3F) or 1 μM epoxomicin (Figure 3G), 2 different proteasome inhibitors. Both prevented the decrease in Ikaros. Conversely, the addition of proteasome inhibitors had no effect on BCL11A protein levels (Figure 3H). Thus, pomalidomide leads to the selective proteasomal degradation of IKZF1 during erythroid differentiation.
Genetic ablation of Ikaros delays erythropoiesis but does not phenocopy pomalidomide treatment
Although several studies have linked Ikaros to HbF production,32,33 a link between Ikaros and BCL11A has never been established. To better understand the role played by Ikaros, we used lentivirally encoded shRNAs to knockdown IKZF1 in culture. Two different shRNAs corresponding to different regions of IKZF1 were used, both efficiently decreasing Ikaros expression by >90% (Figure 4C). IKZF1 knockdown led to a delay in erythropoiesis (Figure 4A). Moreover, IKZF1 knockdown cells exhibited increased nuclear-to-cytoplasmic ratio and decreased enucleation (Figure 4B). However, there was no change in BCL11A expression levels, precluding any regulatory link between Ikaros and BCL11A (Figure 4C).
Loss of IKZF1 alone is insufficient to recapitulate the effects of pomalidomide treatment. CD34+ cells were transduced with either control (luciferase) or IKZF1 lentivirus, and after this were placed in erythroid differentiation media ± 1 μM pomalidomide (N = 3). Puromycin selection was performed at 48 hours post-transduction. (A) Terminal erythroid differentiation was assessed as described. (B) Representative May-Grunwald Giemsa–stained cytopreps, and enucleation rate as determined by Syto-16 staining. (C) Western blots for IKZF1 and BCL11A at day 7 of culture. (D) Terminal differentiation of shIKZF1 knockdown in the presence of 1 μM pomalidomide. (E) Immunoblots for IKZF1 and BCL11A at day 11.
Loss of IKZF1 alone is insufficient to recapitulate the effects of pomalidomide treatment. CD34+ cells were transduced with either control (luciferase) or IKZF1 lentivirus, and after this were placed in erythroid differentiation media ± 1 μM pomalidomide (N = 3). Puromycin selection was performed at 48 hours post-transduction. (A) Terminal erythroid differentiation was assessed as described. (B) Representative May-Grunwald Giemsa–stained cytopreps, and enucleation rate as determined by Syto-16 staining. (C) Western blots for IKZF1 and BCL11A at day 7 of culture. (D) Terminal differentiation of shIKZF1 knockdown in the presence of 1 μM pomalidomide. (E) Immunoblots for IKZF1 and BCL11A at day 11.
Because IKZF1 knockdown alone did not phenocopy pomalidomide, we hypothesized that pathways independent of Ikaros were involved. We therefore performed IKZF1 knockdowns in the presence of 1 μM pomalidomide and evaluated both BCL11A expression levels and F-cell production. The addition of pomalidomide slightly increased the number of GPAneg cells at day 7, but similar to pomalidomide alone, terminal differentiation was not further affected (Figure 4D). Western blot studies revealed that IKZF1 knockdown alone and in the presence of pomalidomide efficiently mitigated Ikaros levels; however, there was an even greater diminution with pomalidomide. Further, BCL11A expression was decreased only in the presence of pomalidomide (Figure 4E). Quantification of F-cells demonstrated that IKZF1 knockdown failed to significantly induce basal F-cell levels and required pomalidomide to stimulate production (supplemental Figure 4).
Pomalidomide partially reprograms adult erythroid progenitors to a fetal-like state
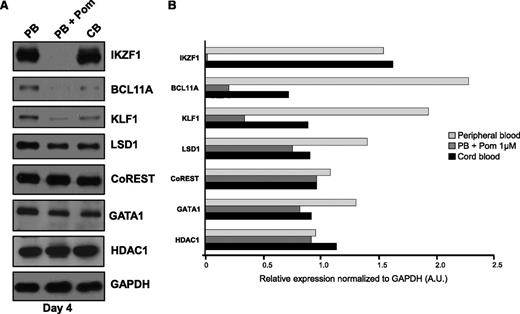
Given the breadth of regulatory molecules targeted by pomalidomide, we posited that the alterations to globin regulatory networks might reflect a reprogramming event whereby pomalidomide induces fetal-like erythropoiesis. To test this, we compared the expression levels of BCL11A, CoREST, GATA1, IKZF1, KLF1, and LSD1 between peripheral adult blood (pomalidomide-treated or not) and cord blood at day 4 of differentiation. Pomalidomide-treated adult cells presented similar expression profiles to that of cord blood–differentiated progenitors (Figure 5A-B), highlighting the specificity for pomalidomide and precluding an artifact as a result of culture conditions. An exception to our findings was IKZF1, for which levels were unchanged in untreated peripheral blood and cord blood cells. Conversely, IKZF1 was virtually absent from pomalidomide-treated conditions, reinforcing the hypothesis that pomalidomide acts through 2 distinct pathways.
Erythropoiesis in pomalidomide-treated conditions is analogous to fetal-state erythropoiesis. (A) Adult peripheral blood or cord blood–derived CD34+ cells were differentiated toward the red cell lineage using the three-phase culture system. Protein lysates were collected at day 4 and immunoblotted for the indicated proteins (N = 3). (B) Quantification of immunoreactive bands normalized to GAPDH.
Erythropoiesis in pomalidomide-treated conditions is analogous to fetal-state erythropoiesis. (A) Adult peripheral blood or cord blood–derived CD34+ cells were differentiated toward the red cell lineage using the three-phase culture system. Protein lysates were collected at day 4 and immunoblotted for the indicated proteins (N = 3). (B) Quantification of immunoreactive bands normalized to GAPDH.
The mechanism of action of pomalidomide is conserved in cells derived from patients with SCD
To determine whether the mechanism of action of pomalidomide was maintained in CD34+ cells derived from patients with SCD, we monitored the effects of pomalidomide on the differentiation of CD34+ progenitors (hemoglobin SS). Cell proliferation was unaffected by 1 μM pomalidomide (Figure 6A). At day 2 of differentiation, increased BFU-Es and decreased CFU-Es were evident in the presence of pomalidomide (Figure 6B). Pomalidomide did not affect terminal differentiation (Figure 6C-D).
The mechanism of action of pomalidomide is conserved in CD34+ cells derived from sickle cell patients. CD34+ cells derived from the peripheral blood of SCD patients were expanded for 4 days and differentiated toward reticulocytes using the three-phase culture system. (A) Growth curves of DMSO and pomalidomide-treated cultures. The data are shown as the mean cells/mL ± SEM (N = 3). (B-C) Flow cytometric analyses of (B) BFU/CFU-E and (C) terminal erythroid differentiation as described in Figure 2 (N = 3). (D) May-Grunwald Giemsa–stained cytopreps at indicated days. Bar represents 5 μm. (E) Western blot for IKZF1 after treatment with DMSO, 1 μM pomalidomide, 5 μM MG132, or the combination of Pom 1μM and MG132 5μM for indicated times (N = 3). (F) Western blot for the expression levels of SOX6, BCL11A, KLF1, and CoREST at day 4 and day 11 (N = 3). (G) Quantification of immunoreactive bands normalized to GAPDH. (H) Immunoblot analyses of α-, β-, and γ-globin chains (N = 3). (I-J) Hemoglobin production was assessed by HPLC using supernatants from dH2O lysed cells. (I) Representative chromatograms from DMSO and pomalidomide-treated cultures. Gray and white arrows indicate peaks corresponding to HbF and HbA, respectively. (J) Quantification of hemoglobin HPLC displayed as the mean percent HbF/(HbF + HbS) ± SEM (**P = .0063; N = 3) and the mean percent HbS/(HbS + HbF) ± SEM (**P = .0127; N = 3). (K) Cellular distribution of HbF by flow cytometry at day 14. The box indicates HbFpos cells (F-cells) (N = 3).
The mechanism of action of pomalidomide is conserved in CD34+ cells derived from sickle cell patients. CD34+ cells derived from the peripheral blood of SCD patients were expanded for 4 days and differentiated toward reticulocytes using the three-phase culture system. (A) Growth curves of DMSO and pomalidomide-treated cultures. The data are shown as the mean cells/mL ± SEM (N = 3). (B-C) Flow cytometric analyses of (B) BFU/CFU-E and (C) terminal erythroid differentiation as described in Figure 2 (N = 3). (D) May-Grunwald Giemsa–stained cytopreps at indicated days. Bar represents 5 μm. (E) Western blot for IKZF1 after treatment with DMSO, 1 μM pomalidomide, 5 μM MG132, or the combination of Pom 1μM and MG132 5μM for indicated times (N = 3). (F) Western blot for the expression levels of SOX6, BCL11A, KLF1, and CoREST at day 4 and day 11 (N = 3). (G) Quantification of immunoreactive bands normalized to GAPDH. (H) Immunoblot analyses of α-, β-, and γ-globin chains (N = 3). (I-J) Hemoglobin production was assessed by HPLC using supernatants from dH2O lysed cells. (I) Representative chromatograms from DMSO and pomalidomide-treated cultures. Gray and white arrows indicate peaks corresponding to HbF and HbA, respectively. (J) Quantification of hemoglobin HPLC displayed as the mean percent HbF/(HbF + HbS) ± SEM (**P = .0063; N = 3) and the mean percent HbS/(HbS + HbF) ± SEM (**P = .0127; N = 3). (K) Cellular distribution of HbF by flow cytometry at day 14. The box indicates HbFpos cells (F-cells) (N = 3).
We then evaluated the expression levels of BCL11A, CoREST, KLF1, and SOX6 as well as Ikaros by western blotting. As in control CD34+ cells, pomalidomide induced the proteasomal degradation of IKZF1 (Figure 6E). Although the expression levels of BCL11A and SOX6 were decreased at day 4 and day 11, KLF1 expression was reduced only at day 4 and CoREST levels remained unaffected, demonstrating similar protein selectivity between control adult and sickle cell–derived progenitors (Figure 6F-G).
Similar to healthy controls, pomalidomide induced γ-globin chain production and decreased β-globin expression, whereas α-globin levels remained unaffected (Figure 6H). Hemolysates revealed an almost fivefold increase in HbF production, and a 40% reduction in HbS (Figure 6I-J). Conversely, HU induced only a twofold increase in HbF, and a nonsignificant reduction in HbS (supplemental Figure 5A-B). However, both pomalidomide and hydroxyurea mediated a pancellular distribution of HbF (Figure 6K and supplemental Figure 5C).
Pomalidomide treatment leads to γ-globin production in MM patients
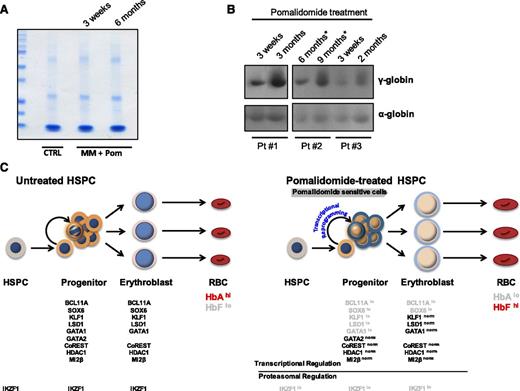
To investigate the effects of pomalidomide in vivo, we analyzed γ-globin levels in MM patients treated with pomalidomide. Because deidentified blood was analyzed, only the duration and dosage of pomalidomide, as indicated, were available. Although the major RBC membrane or cytoskeleton-associated proteins (Figure 7A) and α-globin chains were unchanged, we noted a gradual increase in γ-globin protein expression in RBC lysates of pomalidomide-treated individuals (Figure 7B) that correlated with the length of treatment. These data, which will need to be confirmed in additional cohorts and matched to patients with myeloma before treatment, suggest that pomalidomide may have an in vivo effect for inducing HbF expression in humans.
Red blood cells from MM patients treated with pomalidomide show increased levels of γ-globin and proposed model. Whole-blood samples were obtained from MM patients treated with pomalidomide for varying doses and durations. (A) Coomassie gel staining of normal hematologic control vs MM patients treated with pomalidomide. (B) Expression levels of α- and γ-globin chains in RBCs derived from 3 MM patients treated with pomalidomide for different periods of time. The asterisk next to the time points for patient #2 denotes that the patient interrupted treatment during the time course of the study. (C) Proposed model: In the absence of pomalidomide, adult hematopoietic progenitors differentiate to erythroid progenitors that express BCL11A, SOX6, KLF1, LSD1, GATA1, GATA2, CoREST, HDAC1, and Mi2β. Erythroid progenitors downregulate GATA2 as they progress to terminal erythroblasts. High expression of these factors in erythroid progenitors (bold text) and erythroblasts lead to RBCs containing predominantly HbA with low levels of HbF (shaded text). In the presence of pomalidomide, there is an immediate loss of IKZF1 via proteasomal degradation in HSPCs and erythroid progenitors. However, this post-translational regulation of IKZF1 is independent of the transcriptional/post-transcriptional reprogramming event that we observe in erythroid progenitors, whereby the expression levels of key γ-globin repressors are reduced. This regulation is highly selective because HDAC1, CoREST, GATA2, and mi2β exhibit normal expression. As reprogrammed progenitors mature into erythroblasts, GATA2 is lost, the levels of BCL11A and SOX6 remain decreased, and KLF1, LSD1, and GATA1 expression normalizes. Ultimately, macrocytic (indicated by larger size) RBCs containing high amounts of HbF are produced.
Red blood cells from MM patients treated with pomalidomide show increased levels of γ-globin and proposed model. Whole-blood samples were obtained from MM patients treated with pomalidomide for varying doses and durations. (A) Coomassie gel staining of normal hematologic control vs MM patients treated with pomalidomide. (B) Expression levels of α- and γ-globin chains in RBCs derived from 3 MM patients treated with pomalidomide for different periods of time. The asterisk next to the time points for patient #2 denotes that the patient interrupted treatment during the time course of the study. (C) Proposed model: In the absence of pomalidomide, adult hematopoietic progenitors differentiate to erythroid progenitors that express BCL11A, SOX6, KLF1, LSD1, GATA1, GATA2, CoREST, HDAC1, and Mi2β. Erythroid progenitors downregulate GATA2 as they progress to terminal erythroblasts. High expression of these factors in erythroid progenitors (bold text) and erythroblasts lead to RBCs containing predominantly HbA with low levels of HbF (shaded text). In the presence of pomalidomide, there is an immediate loss of IKZF1 via proteasomal degradation in HSPCs and erythroid progenitors. However, this post-translational regulation of IKZF1 is independent of the transcriptional/post-transcriptional reprogramming event that we observe in erythroid progenitors, whereby the expression levels of key γ-globin repressors are reduced. This regulation is highly selective because HDAC1, CoREST, GATA2, and mi2β exhibit normal expression. As reprogrammed progenitors mature into erythroblasts, GATA2 is lost, the levels of BCL11A and SOX6 remain decreased, and KLF1, LSD1, and GATA1 expression normalizes. Ultimately, macrocytic (indicated by larger size) RBCs containing high amounts of HbF are produced.
Discussion
In this report, we partially identified the mechanism of action of pomalidomide during human erythropoiesis, in normal and sickle cell–derived CD34+ cells differentiated toward reticulocytes (Figure 7C). A previous study reported the observation that pomalidomide induces F-cell production in culture, but did not investigate the mechanism.21 Contrary to HU, pomalidomide induced reciprocal expression of β- and γ-globin genes. The levels of HbF in the pomalidomide-treated cultures were reminiscent of levels observed in the asymptomatic condition of hereditary persistence of HbF, an important therapeutic threshold for any drug to be used to treat SCD.34 We found a transient delay early in differentiation, at the BFU-E to CFU-E transition. Proliferation rates were not affected until this stage, but we noticed a slight increase after day 7, when cells enter terminal differentiation. These results suggest that the observed delay allows for the clonal expansion of CFU-Es, leading to the increased amounts of HbF observed. This is in accordance with Papayannopoulou et al, who proposed that the capacity for γ-globin chain expression was determined at or before the CFU-E stage.35,36 This notion is also strengthened by the observation that cells treated with pomalidomide during the first 7 days of culture were the most responsive to the drug. Nevertheless, terminal erythroid differentiation remains unaffected by the drug, because comparable amounts of reticulocytes were generated over the culture period.
A number of different factors have been implicated in the repression of γ-globin expression. We asked whether these proteins were mechanistically involved in pomalidomide-induced HbF induction. After 4 days of differentiation, transcript and protein levels of the major globin and erythroid factors BCL11A, GATA1, KLF1, LSD1, and SOX6 were reduced. KLF1, GATA1, and LSD1 expression levels normalized as differentiation proceeded; BCL11A and SOX6 expression remained low and CoREST, GATA2, and HDAC1 were unaffected. There was variability in the decrease of expression levels among proteins (eg, KLF-1 compared with GATA-1), suggesting that there is specificity in terms of the mechanism of action of pomalidomide, but also emphasizes the notion that the phenotype observed is not simply caused by delayed maturation in the culture system (ie, CoREST, GATA-2, and HDAC-1 remain unchanged). In addition, quantitative analyses of the western blots revealed some discrepancies between the levels of reduction at the protein levels when compared with the decreases observed by qRT-PCR for the transcripts. These results suggest that multiple levels of regulation are involved in the mechanism of action of pomalidomide, including transcriptional and post-transcriptional events notably for BCL11A. The contribution of the post-transcriptional regulation of BCL11A by pomalidomide will be the focus of future studies.
In contrast, the known mechanism of action of pomalidomide in MM is predominantly post-translational. Indeed, after binding to the E3-ligase cereblon, pomalidomide induces Ikaros degradation by the proteasome and leads to myeloma cytotoxicity18,19 suggesting a role for Ikaros in pomalidomide-induced HbF induction. Furthermore, using a dominant negative form of Ikaros (IK6), Dijon et al demonstrated that the transcription factor plays a role in human erythropoiesis.32 Studies in humanized mice showed that the loss of Ikaros delayed the fetal-to-adult globin switch.33 Therefore, we hypothesized that Ikaros might represent the most upstream target of pomalidomide. However, genetic ablation of Ikaros failed to affect BCL11A expression and HbF production, suggesting that reversal of the adult globin switch by pomalidomide is independent of Ikaros degradation. Indeed, when pomalidomide was added to Ikaros knockdown cultures, BCL11A expression levels and the amount of F-cells produced were comparable with pomalidomide alone. Taken together, pomalidomide achieves its antimyeloma effects and HbF reactivation through divergent mechanisms, specifically independently of proteasome degradation of Ikaros. Our Ikaros knockdown studies challenge the notion that Ikaros plays a substantial role in γ-globin repression in adult erythropoiesis.
Ex vivo differentiation of CD34+ cells to erythrocytes reveals that fetal- and adult-derived progenitors possess different hemoglobin expression patterns and maturation kinetics, suggesting that fetal and adult erythroid characteristics/behaviors are stably programmed in early hematopoietic progenitors. In our studies, pomalidomide appeared to partially revert adult erythroid cells to a fetal-like state with regards to cell proliferation, erythropoiesis, transcription networks expression levels, and hemoglobin production. Analogous to cord blood cells, pomalidomide-treated cultures exhibited a sustained level of cell growth, which was not observed in control peripheral blood–derived cells. In addition, the expression levels of key erythroid and globin gene regulatory proteins were strikingly similar between cord blood–derived and pomalidomide-treated adult cells. Together, these results suggest that pomalidomide reprograms adult erythroid progenitors to differentiate in a manner resembling fetal erythropoiesis. Reprogramming likely occurs early after pomalidomide treatment because changes to BFU-E and CFU-E maturation were seen on day 2 of culture. Further studies are needed to fully characterize this reprogramming event.
The phenotype obtained in normal controls was fully reproduced in CD34+ cells derived from SCD patients. Although the levels of HbF at baseline were higher in these patients, a fourfold increase was observed when the cells were treated with pomalidomide, and a pancellular distribution of HbF was achieved. Both in control and sickle cell–derived cultures, the effect was greater than when the cells were treated with HU. Earlier studies have suggested the use of pomalidomide in combination with hydroxyurea for the treatment of SCD.21 However, when both were administered in the humanized SCD mice models, the effects of each drug were abolished.22 Although the mechanism of action of HU is still not fully understood, it acts, at least partly, by interfering with cell cycle progression.37,38 In our studies, however, pomalidomide does not alter the cell cycle. It is conceivable that HU negates pomalidomide-mediated reprogramming by interfering with the clonal expansion of the precursors.
The effectiveness of pomalidomide in HbF induction has yet to be comprehensively evaluated in humans. Pomalidomide is only approved for the treatment of MM. Significant levels of γ-globin expression were found in MM patients treated with pomalidomide. This suggests that an off target effect of the drug in these patients is to raise the level of HbF, and also brings the proof of concept for the validity of the use of pomalidomide in a clinical trial for the treatment of SCD. It should be noted that the effects observed are not during normal erythropoiesis, but erythropoiesis in the presence of myeloma cells in the marrow. However, the fact that during treatment, the expression of the γ-globin chain was increased in all 3 patients studied suggests that pomalidomide may indeed be effective during normal conditions as well.
Pomalidomide demonstrates excellent blood-brain barrier penetrance in murine models39 and we found that pomalidomide decreases BCL11A expression. This raises the issue of safety in terms of neurogenesis, because patients with deletions in BCL11A appear prone to schizophrenia and autism spectrum disorders.40,41 However, in preliminary studies, pomalidomide was added to human neuronal cell lines, and there was no change in the expression levels of BCL11A (supplemental Figure 6). In addition, pomalidomide induces less neuropathy than thalidomide.17
Our data give insights into the underlying mechanism of HbF induction by pomalidomide and supports the notion that pomalidomide leads to a reprogramming of the adult progenitor cells toward a fetal program (Figure 7C). Because pomalidomide is approved for another indication, and has an acceptable safety profile, our results strengthen the rationale for clinical trials using this drug for hemoglobinopathies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their contribution to this study; and Drs Mitch Weiss, Luanne Peters, Kevin J. Tracey, Betty Diamond, and Bettie Steinberg for helpful discussions and critical reading of the manuscript.
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK26263) (N.M.) and National Heart, Lung, and Blood Institute (HL079571) (J. M. Lipton), a New York State Empire Clinical Research Investigator Program award (J. M. Liu), the Pediatric Cancer Foundation (J. M. Lipton, L.B.), and a Feinstein Institute competitive faculty award (L.B.). B.M.D. is the recipient of an American Society of Hematology Physician-Scientist Career Development Award. L.B. is the recipient of an Allied World St. Baldrick’s Scholar Award.
Authorship
Contribution: B.M.D., A.O.A.-K., J.P., P.G.G., N.M., J. M. Lipton, J. M. Liu, and L.B. designed the research; B.M.D., A.O.A.-K., and J.P. performed most of the experiments; A.O.A.K. and S.A.S. cowrote the IRB protocol; J.H. analyzed RNAseq experiments; M.H. and Y.A.-A. designed and performed the HPLC experiments; S.D., M.G., and S.H.-K. provided technical assistance with the western blots and cell culture; K.W.H.C. provided the pomalidomide and designed part of the research; S.L.A. identified the multiple myeloma patients and provided the samples for the study; P.M. designed and analyzed the experiments related to the effect of pomalidomide in neurons; B.M.D., A.O.A.-K., J.P., J.H., Y.A.-A., A.V., N.T., P.M., X.A., P.G.G., N.M., J. M. Lipton, J. M. Liu, and L.B. analyzed and interpreted the data; L.B. and B.M.D. cowrote the manuscript; A.O.A.-K., S.L.A., N.T., P.M., X.A., P.G.G., N.M., J. M. Lipton, and J. M. Liu edited the manuscript; and all authors read and commented on the final manuscript.
Conflict-of-interest: K.W.H.C. is employed by BioTheryX Inc. The remaining authors declare no competing financial interests.
Correspondence: Lionel Blanc, Laboratory of Developmental Erythropoiesis, The Feinstein Institute for Medical Research, 350 Community Dr, Manhasset, NY 11030; e-mail: lblanc@northwell.edu.
References
Author notes
B.M.D. and A.O.A.-K. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal