The importance of research focused on the final events of atherothrombosis cannot be overestimated. Platelet hyperreactivity leading to thrombosis is the main reason for mortality and morbidity in patients with cardiovascular disease and stroke, which together remain a leading cause of death in developed countries. In this issue of Blood, Shen et al1 establish another functional link between proatherogenic lipoproteins and platelet-mediated thrombus formation with a specific focus on stroke. In their model, the initiating component is L5, the electronegative subfraction of low-density lipoproteins (LDLs), which was shown to be substantially elevated in patients with ischemic stroke. L5 was shown to activate platelets via its receptor, lectin-like oxidized LDL receptor-1 (LOX-1), and αβ amyloid peptide, which together contribute to platelet hyperreactivity and stroke complications.
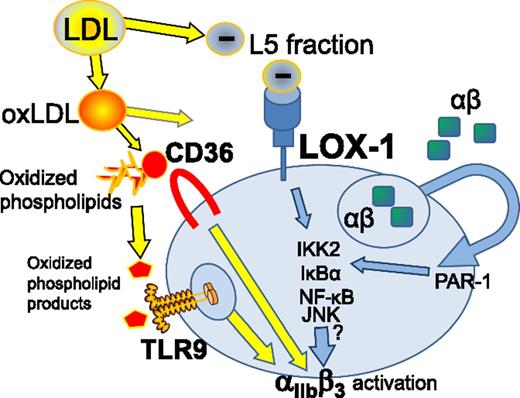
Prothrombotic and proatherogenic LDL fractions and their products operate via platelet pattern recognition receptors, including LOX-1, leading to αIIbβ3 activation and platelet aggregation. JNK, c-Jun N-terminal kinase; oxLDL, oxidized LDL; PAR-1, protease-activated receptor 1; TLR9, Toll-like receptor 9.
Prothrombotic and proatherogenic LDL fractions and their products operate via platelet pattern recognition receptors, including LOX-1, leading to αIIbβ3 activation and platelet aggregation. JNK, c-Jun N-terminal kinase; oxLDL, oxidized LDL; PAR-1, protease-activated receptor 1; TLR9, Toll-like receptor 9.
It is surprising that thrombosis, being the most critical pathological event that underlies the majority of disabilities in United States,2 remains relatively poorly understood. Compared with many other diseases, stroke in general seems to attract less attention from researchers as well as funding agencies.2 While there is a substantial body of literature focused on the role of platelets in cardiovascular complications, the number of articles in PubMed addressing the specifics of platelet activation in stroke is several times lower. The main contributing factor to this phenomenon is, of course, the complexity and multifactorial nature of platelet hyperreactivity.3 Several previous studies have established not only a correlative but also a mechanistic link between hyperlipidemia and thrombosis. Among those are identifications of new interactions between platelet scavenger receptors and well-characterized products of phospholipid oxidation, which lead to platelet aggregation in hyperlipidemia.4 However, the heterogeneity of plasma lipids combined with products of their oxidation is extremely high, therefore suggesting the existence of multiple receptors and mechanisms contributing to platelet activation.
The key role of the electronegative subfraction of LDL is not particularly surprising since high levels of L5 have also been shown to associate with metabolic syndrome and high risk of cardiovascular complications in several pathologies.5 The very same group had previously shown that an L5 subfraction isolated from human patients triggers platelet activation and aggregation via LOX-1 on platelets.6 Highly electronegative LDL from patients with ST-elevation myocardial infarction was shown to trigger platelet activation and aggregation.
In the present study, Shen et al analyzed lipids from ischemic stroke patients to show that, although total cholesterol, LDL, and high-density lipoproteins (HDLs) are similar between the groups, the concentration of L5 is significantly higher than in controls. In agreement, expression levels of the L5 receptor LOX-1 were elevated in platelets from patients with stroke. Then, L5 isolated from these patients was shown to worsen complications in a ligation-induced cerebral ischemia model. Likewise, the blockade of LOX-1 decreased the infarct volume while improving sensorimotor performance in mice. In another model, where thrombosis was induced in the middle cerebral artery, L5 was again thrombogenic, whereas LOX-1 deficiency had an inhibitory effect, thereby solidifying the role of the L5–LOX-1 axis in the pathology of stroke and its complications. Administration of L5 shortened the bleeding times, and LOX-1 blockade restored the tail-bleeding times back to control levels. LOX-1 deficiency only mildly attenuated hemostasis in the absence of L5, thereby demonstrating that normal levels of platelet aggregation remain intact. Another important characteristic of the LOX-1/L5–based blocking approach is that hemorrhaging in the brain was not observed. This is an important point showing a certain potential of this strategy for future prevention of ischemic stroke or acceleration of patients recovery after the event.
One of the connections between stroke pathology and platelet function is uptake and secretion from platelets of amyloid β αβ peptide. Interestingly, platelets serve as one of the main sources of αβ in the circulation.7 Several studies demonstrated that αβ peptide accumulates in the cerebral vasculature with aging and contributes to endothelial damage, which in turn might serve as another pathologic player in a series of events leading to stroke.7 Secretion of αβ amyloid was shown to be inducible by well-known platelet agonists such as thrombin and collagen. Shen et al show that αβ is secreted from platelets activated by L5 via LOX-1 and involves a signaling pathway including IκB kinase 2 (IKK2) and nuclear factor-κB (NF-κB) activation (see figure). This creates an additional stimulatory “autocrine” loop during platelet aggregation. There is an apparent synergism between L5 and the αβ peptide; both are able to augment platelet responses that are induced by their respective “partners in crime.” The clear evidence of NF-κB involvement in platelet responses came from the use of an IKK2 inhibitor in an in vivo hemostasis model. In the presence of both L5 and the αβ amyloid peptide, the IKK2 inhibitor attenuated hemostasis returning bleeding times to control levels. Altogether, this study establishes an important pathway of platelet activation with an apparent relevance to stroke pathobiology.
One of the most remarkable findings of this study is that L5 levels are almost 40-fold higher in stroke patients compared with control subjects, whereas overall LDL and LDL-cholesterol levels are similar. This observation alone points to a potentially key role of this LDL fraction in stroke. The origin of the electronegative fraction of LDL is unknown; however, there is a possibility that the process involves secretory phospholipase A2 and desialylation.8 The properties of negatively charged LDLs, including the tendency to aggregate, promote reactive oxygen species generation and inflammation, are well summarized in a recent review.8 Even after additional separation into L1–L5 fractions, L5 lipoproteins represent a heterogeneous and rather complex mixture. Overall, it remains unknown what exact chemical moieties on L5 lipoproteins are recognized by LOX-1 and stimulate platelet activation.
Taken together with a series of previous reports, it appears that prothrombogenic activities of LDLs are mediated by several pattern recognition receptors expressed on platelets. At present, these include TLR9, a receptor for final oxidized products of phospholipids,9 and 2 distinct scavenger receptors: CD36, a receptor for intermediate products of phospholipid oxidation,3 and LOX-1, a receptor for the negatively charged L5 fraction of LDL.1,6 The involvement of pattern recognition/scavenger receptors is understandable because platelets seem to respond to the rather wide spectrum of compounds. Many of these compounds remain to be identified and fully characterized.
Conflict-of-interest disclosure: The authors declare no competing financial interests.