In this issue of Blood,Chen et al demonstrate that platelets expressing factor VIII (FVIII) shield FVIII from immune detection. In the naive FVIIInull hemophilia A (HA) mouse, platelet-derived VIII prevents both a primary and memory anti-FVIII immune response, and together with total body irradiation, suppresses anti-FVIII immune response.1
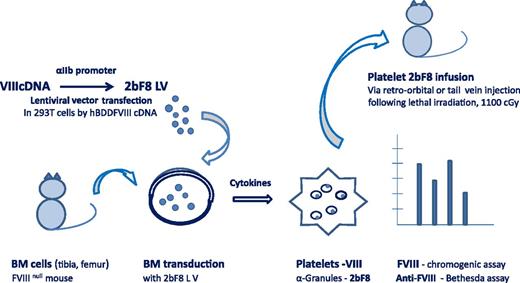
Platelet-derived FVIII. The 2bF8 lentiviral vector (LV) was constructed by transfection of lentivirus in 293T cells by plasmids containing hBDD-FVIII complementary DNA (cDNA). HSCs collected from the bone marrow of tibia and femurs of FVIIInull mice were transduced with 2bF8 LV. Platelets were isolated from HSCs in the presence of cytokines, and 2bF8 LV was detected in platelet α-granules. Following conditioning by a lethal dose of 1100 cGy total body irradiation, 6- to 8-week FVIIInull mice received 2bF8-transduced platelets by retro-orbital or tail-vein injection. Platelet FVIII expression was measured by chromogenic assay and anti-FVIII by Bethesda assay. FVIII function was assessed by survival after tail clip.7-9 BM, bone marrow.
Platelet-derived FVIII. The 2bF8 lentiviral vector (LV) was constructed by transfection of lentivirus in 293T cells by plasmids containing hBDD-FVIII complementary DNA (cDNA). HSCs collected from the bone marrow of tibia and femurs of FVIIInull mice were transduced with 2bF8 LV. Platelets were isolated from HSCs in the presence of cytokines, and 2bF8 LV was detected in platelet α-granules. Following conditioning by a lethal dose of 1100 cGy total body irradiation, 6- to 8-week FVIIInull mice received 2bF8-transduced platelets by retro-orbital or tail-vein injection. Platelet FVIII expression was measured by chromogenic assay and anti-FVIII by Bethesda assay. FVIII function was assessed by survival after tail clip.7-9 BM, bone marrow.
Among the most serious complications of hemophilia treatment is the formation of inhibitor antibodies, which occurs in 25% to 30% of those with severe HA, usually within the first 20 to 30 exposures.2 Inhibitor formation is a T-cell–dependent, B-cell–mediated immune response to foreign infused FVIII,3 which renders standard FVIII replacement therapy ineffective. Treatment is difficult, as bypass agents such as FVIIa or factor-eight inhibitor bypass activity, are less effective than FVIII in noninhibitor patients, and result in poorly controlled bleeding, with 10-fold the cost and twofold the hospitalizations and mortality rate of noninhibitor patients.4 Thus, preventing or reducing inhibitors is a major goal of hemophilia management.
Fortunately, this is an exciting time in hemophilia treatment with the promise of novel therapeutics that potentially bypass existing inhibitor anti-FVIII response, ie, small interfering RNA-antithrombin 3 and bispecific FVIII monoclonal antibody,5 or suppress new anti-FVIII inhibitor response, ie, rFVIIIFc.6 In this study, Chen et al take advantage of their lentiviral-mediated platelet-specific FVIII gene delivery model in which FVIII is protected from protease degradation by storage in platelet α-granules,7-9 to explore the immune response to platelet FVIII (see figure). In their model, hematopoietic stem cells (HSCs) from the FVIIInull HA mouse are transduced ex vivo by lentivirus carrying the 2bF8 transgene (2bF8Tg), with FVIII expression under the platelet-specific glycoprotein αIIb gene promoter. Infusion of genetically modified HSC containing FVIII corrected the bleeding phenotype in the FVIIInull mouse after tail clip, restored therapeutic VIII levels, and also induced FVIII specific tolerance in FVIIInull mice with inhibitors. As myeloablative-preconditioning regimens have been used to create space for engraftment, the authors sought to determine if it had any effect on immune response and, in particular, on immunogenicity of platelet-derived FVIII.
In their current elegant studies, Chen et al showed that infusion of 2bF8 genetically manipulated platelets, ie, platelet-derived FVIII, into the FVIIInull HA mouse not only restores normal hemostasis after tail clip and corrects FVIII levels, but also prevents de novo inhibitor formation. Further, they demonstrated that platelet-derived FVIII prevents a memory immune response to FVIII in FVIIInull mice with inhibitors. Moreover, platelet-derived FVIII suppressed anti-FVIII response even when preconditioning included a nonmyeloablative regimen, thus confirming that platelet-derived FVIII reduces immunogenicity and suppresses anti-VIII response, independent of the effects of a myeloablative conditioning regimen.
So, how does platelet-derived FVIII escape the immune system?
First, these studies indicate that infusion of platelet-derived FVIII is effective against bleeds, and, further, does not trigger an immune response in the FVIIInull HA mouse. The mechanism by which platelet-derived FVIII escapes immune detection is not fully understood. Platelets sequester FVIII in α-granules, and thus FVIII exposure to the blood stream and immune system is very limited. Even in the setting of bleeds, such as during retro-orbital infusion, or endothelial injury when FVIII is released from activated platelets to promote clot formation, or when aged platelets are phagocytosed by macrophages, FVIII would be expected at the site of bleed or injury or phagocytosis in sufficient amounts to trigger the immune system, but no immune response has been detected. The reduced immunogenicity afforded by platelet-derived FVIII appears to be long-lived, as additional experiments have shown anti–FVIII-inhibitor response is suppressed in the HA FVIIInull mouse even weeks after these studies were completed, and after subsequent exposure to standard recombinant FVIII.
Whether platelet-derived FVIII suppresses FVIII-specific T-regulatory cell response is not known, but plausible, as platelets contain many biologically active proteins that are increasingly recognized to play a role in the regulation of immune response in inflammation and infection.10
In conclusion, the transfusion of platelets containing FVIII appears to provide a less immunogenic factor replacement for HA mice. Whether this is also true for patients with HA will require the conduct of prospective clinical trials. If confirmed, this platelet VIII approach may provide a much needed therapeutic alternative to current therapies for hemophilia patients with inhibitors.
Conflict-of-interest disclosure: M.V.R. reports research funding from Alnylam, Baxalta, Bayer, Biogen, Bristol-Myers Squibb, CSL Behring, Dimension Therapeutics, Genentech Roche, Pfizer, SPARK, and Vascular Medicine Institute (University of Pittsburgh); and has received honoraria for consulting for Alnylam, Baxalta, BioMarin, Medscape, Shire, and Tacere Benitec.