Key Points
MDSC treatment prevents GVHD by skewing T cells toward type 2 T cells.
MDSCs proliferate in vivo, suppress independent of major histocompatibility complex class I expression, and do not impair allogeneic T-cell homing and the graft-versus-tumor effect.
Abstract
Myeloid-derived suppressor cells (MDSCs) inhibit T-cell expansion and functions by versatile mechanisms such as nutrient depletion, nitrosylation, or apoptosis. Since graft-versus-host disease (GVHD) is characterized by the expansion of donor-derived T cells destroying recipient tissue, we analyzed whether MDSCs can be used for GVHD prevention in murine allogeneic bone marrow transplantation models. Transplantation of MDSCs, generated from bone marrow cells by granulocyte-macrophage colony-stimulating factor (GM-CSF)/G-CSF in vitro, inhibited GVHD-induced death and attenuated histologic GVHD, whereas antitumor cytotoxicity of alloantigen-specific T cells was maintained. MDSCs expanded in vivo and invaded lymphatic and GVHD target organs. Major histocompatibility complex class I expression on MDSCs was dispensable for their suppressive capacity. Inhibition of GVHD required the presence of MDSCs during T-cell priming, whereas allogeneic T-cell numbers and homing in lymphoid and GVHD target organs were not considerably affected in MDSC-treated mice. However, MDSCs skewed allogeneic T cells toward type 2 T cells upregulating T helper 2 (Th2)-specific cytokines. Type 2 T-cell induction was indispensable for GVHD prevention since MDSC treatment failed to prevent GVHD when allogeneic STAT6-deficient T cells, which are unable to differentiate into Th2 cells, were transplanted. MDSC-induced Th2 induction might be applicable for GVHD treatment in clinical settings.
Introduction
Graft-versus-host disease (GVHD) represents the major complication after allogeneic bone marrow transplantation (BMT). Mature T cells in the transplant activated by alloantigens of the host attack and destroy recipient tissue (ie, GVHD), but are also responsible for the eradication of residual tumor cells (ie, graft-versus-tumor [GVT] effect). Allogeneic T-cell activation and proliferation are attempts to be inhibited by routinely given immunosuppressive therapy, reducing the GVHD risk but mediating general immunosuppression.1,2 Preventing recipient tissue destruction while preserving T-cell immunity to cope with infections and to destroy tumor cells is a major goal in GVHD treatment.
Immune responses are modulated by myeloid-derived suppressor cells (MDSCs), a heterogeneous population of myeloid progenitor cells. In healthy conditions, myeloid progenitors quickly differentiate into mature myeloid cells, whereas pathological conditions block myeloid differentiation and support the expansion of MDSCs.3 Murine MDSCs co-express Gr-1 and CD11b. The 2 major MDSC subsets are characterized by the different expression of the 2 Gr-1 epitopes, Ly-6C and Ly-6G: granulocytic MDSCs are CD11b+Ly-6G+Ly-6Clow, whereas monocytic MDSCs are CD11b+Ly-6G−Ly-6Chigh.4 Immunosuppressive capacity of both subsets is associated with increased expression of arginase-1 and inducible nitric oxide synthetase (iNOS), enzymes depriving l-arginine from the microenvironment, that is required for T-cell proliferation. Nitrosylation of T-cell–associated molecules, interference with T-cell homing, and induction of T-cell apoptosis and regulatory T cells (Tregs) are also reported to be responsible for MDSC-mediated immunosuppression.5 Whether T-cell inhibition by MDSCs requires major histocompatibility complex (MHC)-mediated antigen presentation or is antigen-unspecific is still not definitely clarified. Although MDSCs isolated from tumor-bearing mice solely prevent antigen-specific T-cell responses,6,7 deficiency in MHC class I molecules did not impair the suppressive capacity of MDSCs.7 MDSCs can also influence the cytokine environment. Elevated MDSC numbers in different murine disease models are associated with an increase of T helper 2 (Th2) immunity.8,9 Although the presence of Th2 cells is advantageous for GVHD inhibition, the antitumor effects of type 2 T cells are less pronounced than for type 1 T cells.10-14
MDSCs can be generated in vitro by various methods using different progenitor cells and cytokine combinations for differentiation and expansion.15-19 Usage of MDSCs as cellular therapy was effective for inhibition of experimental autoimmune myasthenia gravis,18 prolonging allograft survival,20 and for GVHD prevention,16,17 clearly indicating that these cells have a therapeutic potential in T-cell–mediated diseases.
In the present study, we show that MDSCs efficiently prevent clinical GVHD by skewing T-cell responses toward type 2 T-cell immunity. Because MDSC cotransplantation does not abrogate the GVT effect, these suppressor cells might provide a treatment option in GVHD prevention.
Methods
Cell culture
Cell lines were grown in RPMI 1640, 10% fetal calf serum (Lonza), 2 mM l-glutamine, 1 mM sodium pyruvat at 37°C, and 7.5% CO2. Primary cells were cultured in α-minimum essential medium, 10% fetal calf serum, 2 mM l-glutamine, 1 mM sodium-pyruvate, 100 U/mL penicillin-streptomycin, and 0.05 mM 2-mercaptoethanol.
MDSC generation
A total of 3 × 105 bone marrow (BM) cells/mL were cultured with granulocyte colony-stimulating factor (G-CSF) (2000 U/mL) and granulocyte-macrophage CSF (GM-CSF) (250 U/mL) (Peprotech) for 4 days.
Mice and BMT
Female C57BL/6 (B6; H-2b, CD45.2), B6D2F1 (H-2bxd, CD45.2) (Janvier, France), B6.129P2-B2mtm1Unc/J (Cl I−/−, H-2b, CD45.2) (The Jackson Laboratory), B6.SJL-PtprcaPepcb/BoyJ (B6.SJL; H-2b, CD45.1), B6.C-H2-Kbm1/ByJ (B6.bm1; H-2Kbm1, CD45.2), and B6.129S2(C)-STAT6tm1Gru/J (STAT6−/−, H-2b, CD45.2) mice (breeding pairs from The Jackson Laboratory and bred at University of Ulm) were used (6 to 12 weeks of age). On day −1, mice were irradiated with 14 Gy split into 2 doses 4 hours apart from a 137Cs source and were reconstituted on day 0 with 5 × 106 IV T-cell depleted BM (TCD-BM). T cells were depleted by incubation 30-H12 supernatant (anti–Thy-1.2) and with subsequent lysis with rabbit complement (Cedarlane). TCD-BM was co-injected with 2 × 107 spleen cells (SCs) and MDSCs. GVHD scores were determined by analyzing weight, activity, skin, fur ruffling, and posture according to Cook et al.21 Animals dying during the experiment remained included in the calculation until the end of the experiment with their final GVHD scores. JM6 (H-2Kbm1, 5 × 104/mouse) were IV injected at the day of BMT. Regierungspräsidium, Tübingen, Germany approved all animal experiments (1031, 1065, and 1084).
Generation of tumor cell line JM6 from B6.bm1 mice
B6.bm1 mice were irradiated 4 times with 1-week intervals with 1.7 Gy.22 Thymomas appearing 6 months later were resected and cultured as single-cell suspensions. Outgrowing tumor cells were cloned 3 times to obtain the stable CD8+ single-cell clone JM6.
Histopathology
Organs were fixed in 4% formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Slides were examined by a pathologist who was blinded for the experimental history. Histopathology of GVHD was graded according to Kaplan et al.23
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeling
A total of 2 × 106 cells were labeled with 100 µL of 50 µM CFSE (eBioscience) for 10 minutes at 37°C, washed, and immediately used for IV injections or in vitro assays.
Mixed lymphocyte reaction
A total of 2.5 × 105 CFSE-labeled SCs were stimulated with 2.5 × 105 irradiated stimulator SCs (33 Gy) in the absence or presence of MDSCs. Arginase-1 was inhibited by N-hydroxy-nor-arginine (nor-NOHA) and iNOS by L-NG-monomethyl-arginine-citrate (L-NMMA) (Merck). Proliferation was determined by flow cytometry. Percentage suppression = 100 − (% proliferating T cells + stimulators + MDSC) / (% proliferating T cells + stimulators) × 100.
Isolation of cells
For information about cell isolation procedures, see supplemental Methods on the Blood Web site.
Interleukin (IL)-2 secretion assay and intracellular staining
SCs were restimulated with medium, phorbol myristate acetate (PMA) (20 ng/mL) plus ionomycin (1 µM) (Calbiochem) or allogeneic SCs (1:1 ratio) for 5 hours. IL-2 secretion was determined by the IL-2 secretion assay (Miltenyi Biotec). Intracellular cytokines were determined by adding 10 µg/mL brefeldin A (Sigma-Aldrich) during the stimulation process. After 5 hours, cells were stained for surface markers, fixed with 4% paraformaldehyde, subsequently lysed with 0.1% saponin (Sigma-Aldrich), and stained for cytokine expression. STAT6 was stained with a fixation/permeabilization solution kit (BD Biosciences).
Serum cytokine analysis
Cytokine serum concentrations were determined using the mouse Th1/Th2/Th17/Th22 13plex FlowCytomix Multiplex Kit (eBioscience), measured on LSR II flow cytometer, and analyzed by eBioscience’s FlowCytomix software.
Flow cytometry
A total of 5 × 105 cells were stained. Antibodies used are specified in supplemental Table 1.
RNA preparation and quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
RNA was isolated using RNeasy Lipid Tissue Mini Kit (Qiagen) and complementary DNA was synthesized using SuperScript II Reverse Transcriptase (Life Technologies). qRT-PCR was performed with a LightCycler 2.0 using a LightCycler FastStart DNA Master PLUS SYBR Green I Kit (Roche Diagnostics). The qRT-PCR results were normalized using mouse aryl hydrocarbon receptor-interacting protein (AIP) as house keeping gene. Primer sets (Thermo Fisher Scientific) used are listed in supplemental Table 2.
Statistics
Survival was analyzed using the Kaplan–Meier method and Log-Rank statistics, and a Student t or analysis of variance test was applied for the other studies. Results were considered significant if P ≤ .05.
Results
In vitro-generated MDSCs prevent GVHD when co-injected with the allogeneic transplant
MDSCs were generated from BM cells by GM-CSF/G-CSF incubation. After 4 days, 92% of the cells expressed CD11b+Gr-1+, and CD11b+ cells can be subdivided in monocytic (Ly-6ChighLy-6G−) and granulocytic MDSCs (Ly-6ClowLy-6G+) (Figure 1A). MDSCs expressed CD115 and CD124, markers probably indicative for their suppressive action, costimulatory molecules CD80 and CD86, and LFA-1 and CD62L, enabling homing into lymphoid organs. Nearly all MDSCs express MHC class I, whereas only a few cells are MHC class II or F4/80 positive (supplemental Figure 1A). Immunosuppressive molecules, arginase-1 and iNOS, were strongly upregulated in MDSCs compared with isolated CD11b+ BM cells (supplemental Figure 1B), whereas COX2, known to be important for the suppressive capacity of human MDSCs, was not induced (data not shown). Most importantly, MDSCs efficiently prevented the proliferation of CD8+ CFSE-labeled SCs from B6.SJL (H-2Kb) mice stimulated with irradiated allogeneic SCs from B6.bm1 mice (H-2Kbm1). MDSCs suppressed T-cell proliferation preferentially by iNOS, since the iNOS inhibitor L-NMMA abrogated the suppressive effect, whereas the arginase-1 inhibitor nor-NOHA was ineffective (Figure 1B).
MDSCs prevent GVHD in an MHC class I mismatched B6 → B6.bm1 BMT model when co-injected with the transplant. (A) MDSCs were generated in vitro from BM cells of B6 mice in the presence of GM-CSF and G-CSF. After 4 days, cells were stained for CD11b and Gr-1 expression or the distribution of MDSC subsets was defined on side scatter + CD11b+ cells by the expression of Ly-6C and Ly-6G. Data show 1 representative fluorescence-activated cell sorter (FACS) staining from 3 independent experiments performed. (B) CFSE-labeled B6.SJL-derived SCs (CD45.1+, H-2Kb) were stimulated with medium or irradiated B6.bm1 (CD45.2+, H-2Kbm1) allogeneic SCs in the presence or absence of B6-derived MDSCs. iNOS inhibitor L-NMMA or arginase-1 inhibitor nor-NOHA was added. After 4 days, cells were stained for CD45.1 CD3, CD4, and CD8. Proliferation of CD45.1+CD3+ CD8+ T cells was analyzed and suppression of proliferation was calculated. Data represents the mean of triplicates ± standard deviation (SD) of 1 representative experiment out of 3 experiments performed. ***P < .001. (C-E) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs and co-injected with 1 × 106, 5 × 106, or 1 × 107 B6-derived MDSCs on the day of transplantation. Survival (C) and GVHD scores (D) were determined. (C) TCD-BM + SC vs TCD-BM + SC + 1 × 106 MDSCs, *P ≤ .05 vs TCD-BM + SC + 5 × 106 MDSCs, *P ≤ .05 vs TCD-BM + SC + 1 × 107 MDSCs, **P ≤ .01. Surviving animals/total animals treated are indicated in brackets. (D) Error bars indicate ± standard error of the mean (SEM). (E) Paraffin sections of ileum and colon (left), liver (middle), and skin (right) of 5 to 9 animals/treatment group were analyzed for histologic signs of GVHD on the day mice were euthanized due to their moribund state or at the end of the experiment, *P ≤ .05; **P ≤ .01. (F) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM and SC from B6 mice; 1 × 107 B6-derived MDSCs were injected either on the day of transplantation (day 0) or 7 days after BMT (day 7) and survival was determined. Surviving animals/total animals treated are indicated in brackets. TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSCs day 0, *P ≤ .05 vs TCD-BM + SC + 1 × 107 MDSCs day 7, P = .49 considered not significant. n.s., not significant.
MDSCs prevent GVHD in an MHC class I mismatched B6 → B6.bm1 BMT model when co-injected with the transplant. (A) MDSCs were generated in vitro from BM cells of B6 mice in the presence of GM-CSF and G-CSF. After 4 days, cells were stained for CD11b and Gr-1 expression or the distribution of MDSC subsets was defined on side scatter + CD11b+ cells by the expression of Ly-6C and Ly-6G. Data show 1 representative fluorescence-activated cell sorter (FACS) staining from 3 independent experiments performed. (B) CFSE-labeled B6.SJL-derived SCs (CD45.1+, H-2Kb) were stimulated with medium or irradiated B6.bm1 (CD45.2+, H-2Kbm1) allogeneic SCs in the presence or absence of B6-derived MDSCs. iNOS inhibitor L-NMMA or arginase-1 inhibitor nor-NOHA was added. After 4 days, cells were stained for CD45.1 CD3, CD4, and CD8. Proliferation of CD45.1+CD3+ CD8+ T cells was analyzed and suppression of proliferation was calculated. Data represents the mean of triplicates ± standard deviation (SD) of 1 representative experiment out of 3 experiments performed. ***P < .001. (C-E) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs and co-injected with 1 × 106, 5 × 106, or 1 × 107 B6-derived MDSCs on the day of transplantation. Survival (C) and GVHD scores (D) were determined. (C) TCD-BM + SC vs TCD-BM + SC + 1 × 106 MDSCs, *P ≤ .05 vs TCD-BM + SC + 5 × 106 MDSCs, *P ≤ .05 vs TCD-BM + SC + 1 × 107 MDSCs, **P ≤ .01. Surviving animals/total animals treated are indicated in brackets. (D) Error bars indicate ± standard error of the mean (SEM). (E) Paraffin sections of ileum and colon (left), liver (middle), and skin (right) of 5 to 9 animals/treatment group were analyzed for histologic signs of GVHD on the day mice were euthanized due to their moribund state or at the end of the experiment, *P ≤ .05; **P ≤ .01. (F) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM and SC from B6 mice; 1 × 107 B6-derived MDSCs were injected either on the day of transplantation (day 0) or 7 days after BMT (day 7) and survival was determined. Surviving animals/total animals treated are indicated in brackets. TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSCs day 0, *P ≤ .05 vs TCD-BM + SC + 1 × 107 MDSCs day 7, P = .49 considered not significant. n.s., not significant.
To test whether MDSCs can be implemented as cellular GVHD therapy in a single MHC class I-disparate BMT model, lethally irradiated B6.bm1 (H-2Kbm1) mice were reconstituted with TCD-BM and SCs from B6 mice (H-2Kb) and increasing numbers of B6-derived MDSCs. Although transplantation of allogeneic BM and SCs induced severe clinical GVHD with high GVHD scores and a lethality of 60%, injection of MDSCs rescued mortality to about 10%, independent of whether 1, 5, or 10 × 106 MDSCs were injected. Lowest GVHD scores were detected with 1 × 107 MDSCs but were still elevated compared with mice receiving only BM that do not develop GVHD (Figure 1C-D). We also analyzed whether MDSCs can prevent GVHD in the BMT model with a 50% MHC class I and II disparity. MDSCs prevented allogeneic proliferation of B6.SJL-derived T cells stimulated with B6D2F1 SCs in vitro (supplemental Figure 2A). MDSC-treatment with 5 or 10 × 106 cells attenuated GVHD-induced death, and GVHD scores in B6D2F1 mice (H-2bxd) transplanted with B6 (H-2b)-derived TCD-BM and SCs from 90% to 30% (supplemental Figure 2B-C). A significant reduction of histologic GVHD was only observed when 1 × 107 MDSCs were transplanted in both BMT models (Figure 1E and supplemental Figure 2D). Lower MDSC numbers only improved survival and clinical scores without improving the histologic score, indicating that mortality and general tissue damage are independently regulated as reported by others.24,25 The following experiments were performed with 1 × 107 MDSCs. To prevent GVHD, MDSCs need to be co-injected with the allogeneic transplant (day 0), since a therapeutic treatment 7 days after BMT (day 7) only slightly improved survival from 38% to 50% (Figure 1F). Altogether, we could show that cotransplantation of MDSCs significantly prevents GVHD if MDSCs are transplanted prophylactically.
MDSC cotransplantation prevents GVHD independent of MHC class I expression and maintains the GVT effect
To analyze whether MDSC-mediated suppression requires MHC class I expression and antigen presentation,26 B6.bm1 mice were transplanted with B6-derived BM cells and SCs, together with MDSCs derived either from B6 mice (wild-type [WT]) or β2 microglobulin-deficient mice lacking MHC class I expression (Cl I−/−). Interestingly, GVHD-induced death was prevented in about 90% of the mice independent of MHC class I expression on MDSCs, whereas untreated mice had a survival rate of 45%. Also, histologic GVHD of the intestine and skin was attenuated by Cl I−/− MDSCs (Figure 2A-C). MDSCs derived from Cl I−/− mice exhibited a comparable phenotype as WT MDSCs and suppressed T-cell proliferation in vitro in an iNOS-dependent manner (supplemental Figure 3A-B).
MDSC-mediated GVHD suppression is independent of MHC class I expression and does not interfere with the GVT effect. (A-C) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs and conjected with 1 × 107 B6-derived WT or MHC Cl I−/− (Cl I−/−) MDSCs. Survival (A) and GVHD-scores (B) were determined and surviving animals/total animals treated are indicated in the survival curve in brackets. (A) TCD-BM + SC vs TCD-BM + SC + 1 × 107 WT MDSC, *P ≤ .05; TCD-BM + SC vs TCD-BM + SC + 1 × 107 Cl I−/− MDSC, *P ≤ .05. (B) Error bars indicate ± SEM. (C) Paraffin sections of ileum and colon (left), liver (middle), and skin (right) of 8 to 10 animals/treatment group were analyzed for histologic signs of GVHD on the day mice were euthanized due to their moribund state or at the end of the experiment, *P ≤ .05, n.s., not significant. (D-F) Lethally irradiated B6.bm1 mice were transplanted with B6-derived TCD-BM in the presence or absence of B6-derived SCs and 1 × 107 B6-derived MDSCs. CD8+ JM6-thymoma cells (H-2Kbm1) were conjected. Mice were analyzed for survival and surviving animals/total animals treated are indicated in brackets. TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSC; ***P ≤ .001 (D). (D) Spleen and liver weights were determined either the day mice were euthanized due to their moribund state or at the end of the experiment; ***P ≤ .001 (E). (F) Presence of tumor cells in BM, liver, and spleen was determined by the expression of CD8. CD4 and CD8 expression was compared with nontransplanted B6.bm1 mice (untreated). FACS analysis is shown for 1 representative mouse out of at least 5 mice analyzed at the end of the experiment or the day mice were euthanized due to their moribund state.
MDSC-mediated GVHD suppression is independent of MHC class I expression and does not interfere with the GVT effect. (A-C) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs and conjected with 1 × 107 B6-derived WT or MHC Cl I−/− (Cl I−/−) MDSCs. Survival (A) and GVHD-scores (B) were determined and surviving animals/total animals treated are indicated in the survival curve in brackets. (A) TCD-BM + SC vs TCD-BM + SC + 1 × 107 WT MDSC, *P ≤ .05; TCD-BM + SC vs TCD-BM + SC + 1 × 107 Cl I−/− MDSC, *P ≤ .05. (B) Error bars indicate ± SEM. (C) Paraffin sections of ileum and colon (left), liver (middle), and skin (right) of 8 to 10 animals/treatment group were analyzed for histologic signs of GVHD on the day mice were euthanized due to their moribund state or at the end of the experiment, *P ≤ .05, n.s., not significant. (D-F) Lethally irradiated B6.bm1 mice were transplanted with B6-derived TCD-BM in the presence or absence of B6-derived SCs and 1 × 107 B6-derived MDSCs. CD8+ JM6-thymoma cells (H-2Kbm1) were conjected. Mice were analyzed for survival and surviving animals/total animals treated are indicated in brackets. TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSC; ***P ≤ .001 (D). (D) Spleen and liver weights were determined either the day mice were euthanized due to their moribund state or at the end of the experiment; ***P ≤ .001 (E). (F) Presence of tumor cells in BM, liver, and spleen was determined by the expression of CD8. CD4 and CD8 expression was compared with nontransplanted B6.bm1 mice (untreated). FACS analysis is shown for 1 representative mouse out of at least 5 mice analyzed at the end of the experiment or the day mice were euthanized due to their moribund state.
To clarify the influence of MDSCs on the GVT effect, B6.bm1 mice were injected with the CD8+CD4− syngeneic thymoma cell line JM6 and reconstituted with BM cells alone, or BM cells and SCs in the presence or absence of MDSCs. Mice receiving only BM cells died around day 21 from tumor development, reflected by strongly increased spleen and liver weights and increased percentages of CD8+ cells in BM, liver, and spleen. Although mice transplanted with allogeneic BM and SCs were tumor free, 70% of the mice died due to GVHD development, reflected by increased GVHD scores (data not shown). Most importantly, mice treated with MDSCs were tumor free (Figure 2D-F). Allogeneic T cells isolated from MDSC-treated and untreated mice exhibited similar expression of granzyme B and perforin known to be the primary cytotoxic molecules of T-cell–induced tumor destruction27 (supplemental Figure 4). In the B6 → B6D2F1 BMT model, primary Bcr-Abl expressing B-acute lymphoblastic leukemia cells were also efficiently eradicated in MDSC-treated mice reconstituted with allogeneic transplant (supplemental Figure 5A-B), showing that the GVT effect is maintained after MDSC treatment in 2 BMT models.
MDSCs proliferate in vivo, reduce alloantigen-specific T-cell proliferation only insubstantially, and do not influence alloantigen-specific T-cell homing
Because a single MDSC injection inhibited GVHD, in vivo proliferation of MDSCs was analyzed by reconstituting B6.bm1 (CD45.2) mice with B6-derived TCD-BM and SCs (CD45.2) and CFSE-labeled MDSCs derived from B6.SJL mice (CD45.1). One day after transplantation, CD45.1+ MDSCs divided in lymphoid organs and on day 3 extensive proliferation was detected in lymphoid organs and the liver (Figure 3A). Until day 30, transplanted CD45.1+ MDSCs were detectable in the circulation, lymphoid organs, and the liver. Monocytic MDSCs were the major population in the liver, whereas granulocytic MDSCs predominated the lymphoid compartment (supplemental Figure 6A-B).
In vitro-generated MDSCs proliferate in vivo, reduce alloantigen-specific T-cell proliferation only early after transplantation, and do not interfere with allogeneic T-cell homing. (A) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with BM and SCs from B6 mice (H-2Kb, CD45.2+) and co-injected with 1 × 107 CFSE labeled B6.SJL-derived MDSCs (H-2Kb, CD45.1+). Different days after transplantation, mice were euthanized, stained for CD45.1, and proliferation of transplanted CD45.1+ MDSCs was analyzed by CFSE dilution in BM (top), spleen (middle), and liver (bottom) cells. (B-D) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with BM from B6 mice (H-2Kb, CD45.2+) plus B6.SJL-derived SCs (H-2Kb, CD45.1+) in the presence or absence of 1 × 107 B6-derived MDSCs (H-2Kb, CD45.2+). One, 3, and 11 days after BMT, mice were euthanized, SCs stained for CD45.1 and CD3, and numbers of CD45.1+CD3+ T cells were determined (B). Different days after transplantation, spleen, blood, liver, and colon cells were stained for CD45.1 and CD3, and numbers of infiltrating CD45.1+CD3+ T cells were determined in spleen and liver, whereas the percentage of CD45.1+CD3+ T cells was calculated in the blood and colon (C). SCs of mice receiving allogeneic BM and SCs in the presence or absence of MDSCs were isolated 7 days after transplantation and re-stimulated in vitro with medium, PMA/iono or allogeneic B6.bm1 SCs (allo). After 5 hours, the percentage of IL-2–secreting CD45.1+ T cells was determined (D). FACS diagrams are representative for 1 mouse out of 5 analyzed (A). Data represent the mean value ± SD of 5 mice analyzed in (B), of 3 mice analyzed in (C), and of triplicates from SCs, which were pooled from spleens of 7 mice treated in (D); *P ≤ .05; **P ≤ .01. n.s., not significant. No significant statistical differences were detected in T-cell numbers between TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSCs in (C).
In vitro-generated MDSCs proliferate in vivo, reduce alloantigen-specific T-cell proliferation only early after transplantation, and do not interfere with allogeneic T-cell homing. (A) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with BM and SCs from B6 mice (H-2Kb, CD45.2+) and co-injected with 1 × 107 CFSE labeled B6.SJL-derived MDSCs (H-2Kb, CD45.1+). Different days after transplantation, mice were euthanized, stained for CD45.1, and proliferation of transplanted CD45.1+ MDSCs was analyzed by CFSE dilution in BM (top), spleen (middle), and liver (bottom) cells. (B-D) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with BM from B6 mice (H-2Kb, CD45.2+) plus B6.SJL-derived SCs (H-2Kb, CD45.1+) in the presence or absence of 1 × 107 B6-derived MDSCs (H-2Kb, CD45.2+). One, 3, and 11 days after BMT, mice were euthanized, SCs stained for CD45.1 and CD3, and numbers of CD45.1+CD3+ T cells were determined (B). Different days after transplantation, spleen, blood, liver, and colon cells were stained for CD45.1 and CD3, and numbers of infiltrating CD45.1+CD3+ T cells were determined in spleen and liver, whereas the percentage of CD45.1+CD3+ T cells was calculated in the blood and colon (C). SCs of mice receiving allogeneic BM and SCs in the presence or absence of MDSCs were isolated 7 days after transplantation and re-stimulated in vitro with medium, PMA/iono or allogeneic B6.bm1 SCs (allo). After 5 hours, the percentage of IL-2–secreting CD45.1+ T cells was determined (D). FACS diagrams are representative for 1 mouse out of 5 analyzed (A). Data represent the mean value ± SD of 5 mice analyzed in (B), of 3 mice analyzed in (C), and of triplicates from SCs, which were pooled from spleens of 7 mice treated in (D); *P ≤ .05; **P ≤ .01. n.s., not significant. No significant statistical differences were detected in T-cell numbers between TCD-BM + SC vs TCD-BM + SC + 1 × 107 MDSCs in (C).
To clarify how MDSCs prevent GVHD, we first determined whether alloantigen-specific T-cell expansion was inhibited by reconstituting B6.bm1 (CD45.2+) mice with B6-BM (CD45.2+) and B6.SJL-SCs (CD45.1+) in the presence or absence of B6-MDSCs. Numbers of CD45.1+ splenic T cells in MDSC-treated mice were reduced at day 1 and 3 after BMT compared with untreated mice, but were comparable in both groups at day 11 (Figure 3B). Accordingly, alloantigen-specific CD8+ T cells from both groups did not exhibit differences in the development of TEff cells or the expression of activation markers or homing molecules (supplemental Figure 7). No influence of MDSCs on allogeneic T-cell homing was detected because MDSC-treated B6.bm1 mice exhibited no differences in the absolute numbers or percentages of infiltrating CD3+CD45.1+ T cells in the spleen, blood, liver, or colon at different days after BMT (Figure 3C). However, MDSC treatment reduced the amount of IL-2 secreting alloantigen-specific T cells. After re-stimulation of SCs isolated from MDSC-treated B6.bm1 mice with PMA/ionomycin (PMA/iono) or B6.bm1 SCs (allo), the percentage of IL-2 secreting CD45.1+CD8+ T cells was significantly reduced compared with untreated mice independent of the activation signal (Figure 3D). Thus, MDSCs expand in vivo and decrease the amount of IL-2–secreting allogeneic T cells, whereas allogeneic T-cell proliferation or homing is not significantly influenced.
MDSC treatment induces the induction of type 2 T cells
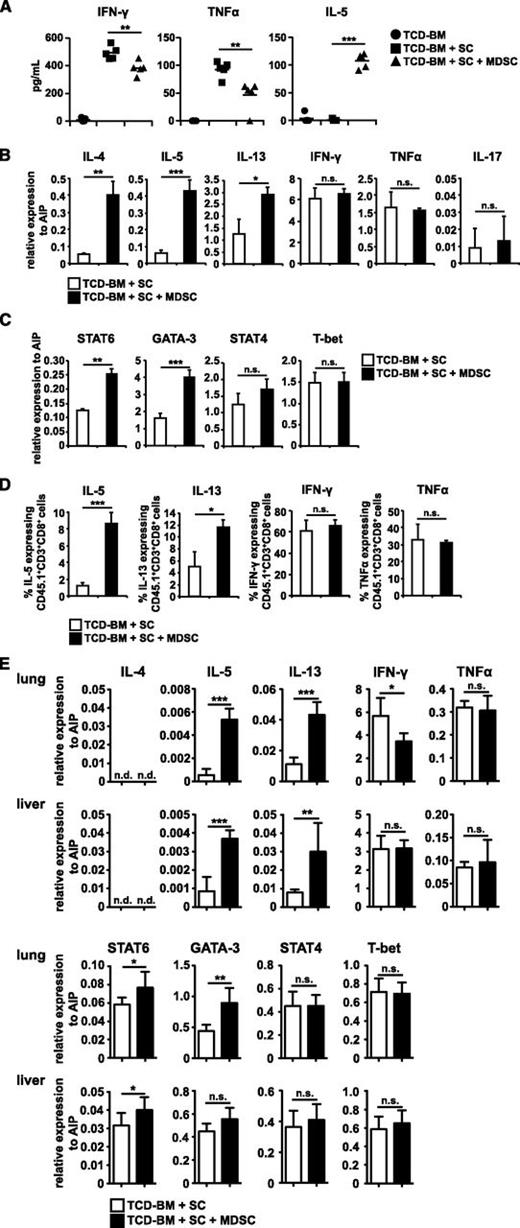
Because IL-2 supports Th1 differentiation,28 serum Th1/Th2 cytokines of transplanted mice were determined. At day 10 after BMT, GVHD-associated Th1 cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α were strongly increased in mice developing GVHD. Importantly, cotransplantation of MDSCs significantly decreased both cytokines. Simultaneously, Th2 serum cytokine concentration of IL-5 was strongly elevated in mice treated with MDSCs compared with untreated mice (Figure 4A). Similar changes in serum cytokines were also detected in the B6 → B6D2F1 model (supplemental Figure 2E). To further prove MDSC-mediated type 2 polarization, messenger RNA (mRNA) expression of alloantigen-specific CD45.1+ T cells isolated from mice reconstituted with allogeneic transplant in the presence or absence of MDSCs were determined (85% CD8+, 15% CD4+). mRNA levels of IL-4, -5, and -13 were strongly upregulated in T cells from MDSC-treated mice (Figure 4B). PMA/iono re-stimulation of SCs confirmed the induction of type 2 T cells as the percentage of IL-5 and -13 producing CD8+ T cells increased in MDSC-treated mice (Figure 4D). Although serum concentrations of IFN-γ and TNF-α were decreased in MDSC-treated mice, no differences in mRNA expression and the percentage of cytokine expressing cells were detected. mRNA expression of IL-17 was also not altered (Figure 4B). Because type 2 T-cell responses require IL-4, STAT6, and GATA-3 expression, whereas type 1 T-cell responses depend on IL-12, STAT4, and T-bet,29 mRNA expression of the transcription factors was determined in CD45.1+ allogeneic T cells. STAT6 and GATA-3 mRNA expression was upregulated in the presence of MDSCs, whereas STAT4 and T-bet expression was unchanged (Figure 4C). STAT6 and GATA-3 protein levels, however, were only marginally increased (data not shown). Most importantly, Th2 skewing in MDSC-treated mice was also observed in allogeneic T cells isolated from GVHD target organs such as lung and liver (Figure 4E).
MDSC cotransplantation skews T cells towards a type 2 phenotype. (A) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice with or without B6-derived SCs in the presence or absence of 1 × 107 B6-derived MDSCs. TNF-α, IFN-γ, and IL-5 concentrations were determined in the serum of transplanted animals 10 days after BMT. (B-E) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with TCD-BM from B6 mice (H-2Kb, CD45.2+) plus B6.SJL-derived SCs (H-2Kb, CD45.1+) in the presence or absence of 1 × 107 B6-derived MDSCs (H-2Kb, CD45.2+). Ten days after transplantation, mice were euthanized and CD45.1+ allogeneic T cells were isolated. qRT-PCRs for the expression of cytokines (B) and transcription factors (C) in allogeneic T cells were performed, and relative expression to AIP was calculated. Ten days after BMT, SCs from mice reconstituted with allogeneic BM and SCs in the presence or absence of MDSCs were re-stimulated with PMA/iono. Cells were stained for surface markers CD45.1, CD3, CD4, and CD8. Percentage of CD45.1+CD3+CD8+ T cells expressing IL-5, IL-13, IFN-γ, and TNF-α was determined by intracellular stainings (D). Ten days after transplantation, mice were euthanized and CD45.1+ allogeneic T cells were isolated from lung and liver. qRT-PCRs for the expression of IL-4, -5, -13, IFN-γ, TNF-α (top) and STAT6, GATA-3, STAT4, and T-bet (bottom) were performed, and relative expression to AIP was calculated (E). Data represent the mean value ± SD of triplicates from spleens of at least 5 mice pooled (B-C). (D) Shows the mean value ± SD of 3 experiments with SCs pooled from 3 mice. (E) Shows the mean value ± SD of 2 experiments with lung and liver cells pooled from at least 6 mice. *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
MDSC cotransplantation skews T cells towards a type 2 phenotype. (A) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice with or without B6-derived SCs in the presence or absence of 1 × 107 B6-derived MDSCs. TNF-α, IFN-γ, and IL-5 concentrations were determined in the serum of transplanted animals 10 days after BMT. (B-E) Lethally irradiated B6.bm1 recipient mice (H-2Kbm1, CD45.2+) were reconstituted with TCD-BM from B6 mice (H-2Kb, CD45.2+) plus B6.SJL-derived SCs (H-2Kb, CD45.1+) in the presence or absence of 1 × 107 B6-derived MDSCs (H-2Kb, CD45.2+). Ten days after transplantation, mice were euthanized and CD45.1+ allogeneic T cells were isolated. qRT-PCRs for the expression of cytokines (B) and transcription factors (C) in allogeneic T cells were performed, and relative expression to AIP was calculated. Ten days after BMT, SCs from mice reconstituted with allogeneic BM and SCs in the presence or absence of MDSCs were re-stimulated with PMA/iono. Cells were stained for surface markers CD45.1, CD3, CD4, and CD8. Percentage of CD45.1+CD3+CD8+ T cells expressing IL-5, IL-13, IFN-γ, and TNF-α was determined by intracellular stainings (D). Ten days after transplantation, mice were euthanized and CD45.1+ allogeneic T cells were isolated from lung and liver. qRT-PCRs for the expression of IL-4, -5, -13, IFN-γ, TNF-α (top) and STAT6, GATA-3, STAT4, and T-bet (bottom) were performed, and relative expression to AIP was calculated (E). Data represent the mean value ± SD of triplicates from spleens of at least 5 mice pooled (B-C). (D) Shows the mean value ± SD of 3 experiments with SCs pooled from 3 mice. (E) Shows the mean value ± SD of 2 experiments with lung and liver cells pooled from at least 6 mice. *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
To prove that MDSC-mediated T-cell polarization is indispensable for GVHD prevention, mice were transplanted with allogeneic STAT6-deficient T cells, which are unable to differentiate into type 2 T cells. Allogeneic STAT6−/− T cells induced GVHD with similar kinetics than WT T cells. Most importantly, cotransplantation of MDSCs could not prevent GVHD-induced death and did not attenuate GVHD scores (Figure 5A-B). Accordingly, serum levels of IFN-γ and TNF-α were not reduced and IL-5 levels were not increased in MDSC-treated mice receiving STAT6−/− T cells (Figure 5C). Ineffectiveness of MDSC treatment in STAT6−/−-transplanted mice is not due to elevated serum levels of GVHD inducing Th1 cytokines IFN-γ or TNF-α, or to an altered MDSC expansion or homing compared with WT T cells (supplemental Figure 8A-C). Allogeneic T cells isolated from mice transplanted with STAT6−/− T cells and MDSCs exhibited no increase in IL-4, -5, and -13 mRNA expression and showed no changes in IFN-γ and TNF-α mRNA expression compared with untreated mice. Interestingly, reconstitution with STAT6−/− T cells increased IL-17 mRNA, which was reduced after MDSC treatment (Figure 5D). STAT6−/− T cells infiltrated the spleen, blood, liver, and colon comparable to WT T cells, independent of the presence of MDSCs (Figure 5E). Thus, MDSC treatment induces a type 2 T-cell response that is indispensable for GVHD prevention.
Induction of type 2 T cells is required for MDSC-mediated GVHD suppression. (A-D) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs or SCs derived from STAT6−/− animals in the absence or presence of 1 × 107 B6-derived MDSCs. Survival (A) and GVHD scores (B) were determined. (A) TCD-BM + SC (B6) vs TCD-BM + SC (B6) + 1 × 107 MDSCs, *P ≤ .05; TCD-BM + SC (STAT6−/−) vs TCD-BM + SC (STAT6−/−) + 1 × 107 MDSCs, P = .94. Surviving animals/total animals treated are indicated in brackets. (B) Error bars indicate ± SEM. TNF-α, IFN-γ, and IL-5 concentrations were determined in the serum of transplanted animals 10 days after BMT (C). Ten days after transplantation, mice were euthanized and CD3+ T cells were isolated. qRT-PCRs for the expression of cytokines IL-4, -5, -13, -17, TNF-α, and IFN-γ were performed, and relative expression to AIP was calculated (D). (E) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice, together with B6.SJL-derived SCs or SCs derived from STAT6−/− animals, in the absence or presence of 1 × 107 B6-derived MDSCs. Different days after BMT, spleen, blood, liver, and colon cells were analyzed for numbers of infiltrating CD3+ T cells in spleen and liver, whereas the percentage of CD3+ T cells was calculated in blood and colon. B6.SJL-derived allogeneic T cells were detected by gating on CD45.1+CD3+ T cells, whereas STAT6−/−-derived allogeneic T cells were detected by gating on STAT6−/−CD3+ T cells. Data represent the mean value ± SD analyzed of triplicates from spleens of at least 5 mice pooled (D) or of 3 mice analyzed on each time point (E). *P ≤ .05; **P ≤ .01; n.s. = not significant. No significant statistical differences were detected in T-cell numbers between the different treatment groups (E). n.s, not significant.
Induction of type 2 T cells is required for MDSC-mediated GVHD suppression. (A-D) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice without or with B6-derived SCs or SCs derived from STAT6−/− animals in the absence or presence of 1 × 107 B6-derived MDSCs. Survival (A) and GVHD scores (B) were determined. (A) TCD-BM + SC (B6) vs TCD-BM + SC (B6) + 1 × 107 MDSCs, *P ≤ .05; TCD-BM + SC (STAT6−/−) vs TCD-BM + SC (STAT6−/−) + 1 × 107 MDSCs, P = .94. Surviving animals/total animals treated are indicated in brackets. (B) Error bars indicate ± SEM. TNF-α, IFN-γ, and IL-5 concentrations were determined in the serum of transplanted animals 10 days after BMT (C). Ten days after transplantation, mice were euthanized and CD3+ T cells were isolated. qRT-PCRs for the expression of cytokines IL-4, -5, -13, -17, TNF-α, and IFN-γ were performed, and relative expression to AIP was calculated (D). (E) Lethally irradiated B6.bm1 recipient mice were reconstituted with TCD-BM from B6 mice, together with B6.SJL-derived SCs or SCs derived from STAT6−/− animals, in the absence or presence of 1 × 107 B6-derived MDSCs. Different days after BMT, spleen, blood, liver, and colon cells were analyzed for numbers of infiltrating CD3+ T cells in spleen and liver, whereas the percentage of CD3+ T cells was calculated in blood and colon. B6.SJL-derived allogeneic T cells were detected by gating on CD45.1+CD3+ T cells, whereas STAT6−/−-derived allogeneic T cells were detected by gating on STAT6−/−CD3+ T cells. Data represent the mean value ± SD analyzed of triplicates from spleens of at least 5 mice pooled (D) or of 3 mice analyzed on each time point (E). *P ≤ .05; **P ≤ .01; n.s. = not significant. No significant statistical differences were detected in T-cell numbers between the different treatment groups (E). n.s, not significant.
A CD11b+CD11c+Gr-1medCD301b+I-Ab+ MDSC subpopulation expands under GVHD conditions in vitro
To further investigate the Th2-inducing capacity of MDSCs, we incubated in vitro-generated MDSCs (day 4 MDSCs) for 3 days with medium supplemented with 2,5% serum derived from GVHD mice (GVHD-MDSC) or with serum derived from mice transplanted with BM cells alone (BM-MDSC). Day 4 MDSCs exhibited a mixed phenotype of CD11b+CD11c− (81%) and CD11b+CD11c+ (16%) cells. After incubation with GVHD medium, the percentage and total numbers of CD11b+CD11c+ cells increased and the percentage of CD11b+CD11c− cells decreased compared with BM-MDSCs (Figure 6A). CD11b+CD11c+ cells of GVHD-MDSCs expressed CD301b, F4/80, I-Ab, and Gr-1med, whereas CD11b+CD11c− cells were CD301b−, F4/80−, I-Ab low, and Gr-1hi (Figure 6B). Surface molecule expression of subpopulations was identical in BM-MDSCs and day 4 MDSCs (data not shown). Because CD301b expression on dendritic cells (DCs) is associated with Th2 induction,30,31 the expression of transcription factors associated with Th2 (IRF4, Klf4)32,33 and Th1 (Batf3)34,35 induction was determined. CD11b+CD11c− cells always express less Klf4 and IRF4 than CD11b+CD11c+ cells in day 4 MDSCs and GVHD-MDSCs. Interestingly, Th1-associated Batf3 expression in CD11b+CD11c+ cells is significantly lower in GVHD-MDSCs compared with day 4 MDSCs (Figure 6C). These data suggest that under GVHD conditions, an antigen-presenting cell-like MDSC subpopulation is developing, which might be responsible for Th2 polarization.
A CD11b+CD11c+Gr-1medCD301b+I-Ab+ MDSC subpopulation expands under GVHD conditions in vitro. (A-C) B6-derived MDSCs were generated in vitro in the presence of GM-CSF and G-CSF. After 4 days, in vitro-generated MDSCs (day4-MDSCs) were incubated for 3 days with medium supplemented with 2.5% serum from mice receiving either TCD-BM alone (BM-MDSCs) or TCD-BM plus SCs (GVHD-MDSCs). Cells were stained for CD11b and CD11c expression before and after serum incubation, and percentage and absolute numbers of CD11b+CD11c− and CD11b+CD11c+ cells were determined. Data represent the mean of triplicates ± SD of 1 representative experiment out of 3 experiments performed (A). Cells were stained for CD11b and CD11c, and the expression of CD301b, F4/80, I-Ab, and Gr-1 was determined on CD11b+CD11c− and CD11b+CD11c+ subpopulations 3 days after serum incubation. Data show 1 representative FACS staining from 3 independent experiments performed. Isotype staining is shown for the CD11b+CD11c+ population but was identical in the CD11b+CD11c− population (B). CD11b+CD11c− and CD11b+CD11c+ cells were isolated from day 4 MDSCs and GVHD-MDSCs, and mRNA expression of Klf4, IRF4, and Batf3 was determined, and relative expression to AIP was calculated. Data represent the mean ± SD of 3 independent experiments performed (C). *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
A CD11b+CD11c+Gr-1medCD301b+I-Ab+ MDSC subpopulation expands under GVHD conditions in vitro. (A-C) B6-derived MDSCs were generated in vitro in the presence of GM-CSF and G-CSF. After 4 days, in vitro-generated MDSCs (day4-MDSCs) were incubated for 3 days with medium supplemented with 2.5% serum from mice receiving either TCD-BM alone (BM-MDSCs) or TCD-BM plus SCs (GVHD-MDSCs). Cells were stained for CD11b and CD11c expression before and after serum incubation, and percentage and absolute numbers of CD11b+CD11c− and CD11b+CD11c+ cells were determined. Data represent the mean of triplicates ± SD of 1 representative experiment out of 3 experiments performed (A). Cells were stained for CD11b and CD11c, and the expression of CD301b, F4/80, I-Ab, and Gr-1 was determined on CD11b+CD11c− and CD11b+CD11c+ subpopulations 3 days after serum incubation. Data show 1 representative FACS staining from 3 independent experiments performed. Isotype staining is shown for the CD11b+CD11c+ population but was identical in the CD11b+CD11c− population (B). CD11b+CD11c− and CD11b+CD11c+ cells were isolated from day 4 MDSCs and GVHD-MDSCs, and mRNA expression of Klf4, IRF4, and Batf3 was determined, and relative expression to AIP was calculated. Data represent the mean ± SD of 3 independent experiments performed (C). *P ≤ .05; **P ≤ .01; ***P ≤ .001. n.s., not significant.
Discussion
GVHD development is characterized by the expansion and the deleterious functions of donor-derived T cells destroying recipient target tissues. To our knowledge, we show for the first time, that co-transplantation of in vitro-generated MDSCs abrogates destructive alloantigen-specific T-cell functions by skewing T cells into type 2 T cells and thereby preventing GVHD, although not abrogating the GVT effect.
MDSCs generated in vitro by incubating BM cells with GM-CSF/G-CSF for 4 days exhibited a CD11b+Gr-1+ phenotype and suppressed T-cell proliferation in vitro in an iNOS-dependent manner. MDSC treatment induced nearly 100% survival in the B6 → B6.bm1 model (difference in one MHC class I molecule) and increased survival from 10% to 70% in the B6 → B6D2F1 model (differences in 50% of MHC class I and II molecules). Efficiency of MDSC treatment might have 2 explanations. First, the success of MDSC treatment might be controlled by the GVHD severity induced, which is dependent on the amount of disparate MHC molecules. Second, CD8+ T cells might be more efficiently suppressed than CD4+ T cells because GVHD in the B6 → B6.bm1 model is dependent on CD8+ T-cell expansion, whereas CD4+ T cells are the GVHD inducers in the B6 → B6D2F1 model.36,37 Preferential blockade of CD8 functions might be due to the high expression of MHC class I on MDSCs compared with class II, which would point to antigen-specific suppression involving antigen uptake, MHC presentation, and interaction with antigen-specific T cells. Antigen-dependent suppression was reported for MDSCs isolated from tumor-bearing mice blocking T-cell responses to MHC class I-specific peptides but failed to inhibit T-cell responses induced by Con A or a MHC class II-specific peptide. Also, the masking of MHC class I molecules on MDSCs abrogated immune suppression by MDSCs.6 However, MHC Cl I−/− MDSCs arising from tumors grown in β2-microglobulin–deficient mice did not exhibit any impairment in inhibiting T-cell proliferation in vitro.7 Because transplantation of MHC Cl I−/− MDSCs did prevent GVHD, MDSC suppression is independent of MHC class I-mediated antigen presentation and depends rather on GVHD severity. MDSC treatment requires prophylactic coinjection with the allogeneic transplant. Multiple MDSC injections after GVHD establishment will show whether MDSCs can also be used therapeutically. Although MDSC function is often associated with activation of Tregs,38,39 Tregs were not increased in MDSC-treated mice (data not shown).
Of note, transplanted MDSCs further expanded after transplantation in lymphoid and GVHD target organs. These results are surprising because apoptosis of CD95+ MDSCs by CD95L-expressing T cells or myeloid cells was reported in murine tumor models,40,41 suggesting that transplanted MDSCs become apoptosis resistant or do not have direct contact with T cells, which express CD95L constitutively. This is further supported by the observation that no significant reduction of alloantigen-specific T cells is detected in mice treated with MDSCs. However, T cells exhibited a different T-cell polarization in the presence of MDSCs. MDSC treatment increased the amount of type 2 T cells and Th2-specific cytokines, although the induction of type 1 T cells was not completely inhibited. Impairing Th2 differentiation by transplanting STAT6−/− allogeneic T cells abrogated the therapeutic effect of MDSCs completely. The association between MDSC induction and the prevalence of Th2 cells was also reported in a sepsis model, in influenza virus-infected mice8,9 and patients with esophageal, pancreatic, or gastric cancer.42,43 In murine asthma models, however, MDSCs shift the balance toward Th1 by blunting the ability of lung DCs to induce re-activation of effector Th2 cells.44,45 Crosstalk of tumor-derived MDSCs with macrophages on the other hand, induces a Th2 response by decreasing macrophage-produced IL-12,46 indicating that the source of MDSCs used and the inflammatory status of the target organ might influence the type of T-cell response initiated. Medium supplemented with serum from GVHD-developing mice induced the outgrowth of a CD11b+CD11c+Gr-1+ MDSC subpopulation expressing surface molecule CD301b and transcription factors IRF4 and Klf4, whose expression was mandatory on DCs for Th2 responses.30-33 CD11b+CD11c+ DCs were also described to exhibit immunosuppressive and tolerogenic characteristics.47,48 Advanced experiments are required to define whether a CD11b+CD11c+Gr-1+ MDSC subpopulation develops in BM-transplanted animals after MDSC treatment in vivo, and to determine its possible role in initiating a Th2 response.
A polarization toward type 2 T-cell immunity is positively associated with attenuated murine GVHD.10,49 Mice transplanted with allogeneic STAT6−/− T cells (and therefore unable to develop Th2 cells) exhibit accelerated GVHD development compared with mice reconstituted with STAT4−/− T cells, which are defective in Th1 development.50 Also, the transfer of in vitro or in vivo polarized type 2 alloreactive CD4+ (Th2) and CD8+ (Tc2) donor T cells attenuates GVHD.12,51 In human GVHD, Th1 and Th2 subsets are described to be equally induced in acute and chronic GVHD and decreased when GVHD resolved.52 However, treatment with the Janus kinase 1/2 selective inhibitor ruxolitinib impairs Th1/17 differentiation, and thereby strongly reduces steroid-refractory human GVHD.53 Dampened Th1/17 responses are also observed in patients receiving extracorporeal photopheresis, which strongly induces MDSC expansion.54 Antitumor cytotoxicity, however, is not abrogated in the presence of Tc2 cells or in mice defective for Th1/Th17 development.13,49 Likewise, antitumor cytotoxicity toward thymomas or Bcr-Abl transduced primary B-acute lymphoblastic leukemia cells was maintained in MDSC-treated mice.
Highfill et al16 identify l-arginine depletion and subsequent reduction of alloantigen-specific T-cell numbers as the major mechanism of MDSC-induced GVHD prevention. In addition to GM-CSF/G-CSF, they use IL-13, known to increase arginase-1 expression55 for MDSC generation, indicating that the cytokines used for MDSC establishment might influence the mode by which MDSCs execute their immunosuppressive function. Alloantigen-specific T-cell proliferation and allogeneic T-cell phenotype in vivo, however, is only determined until day 4 after BMT, while later time points and T-cell polarization are not analyzed. Comparable to our results, the transplanted MDSC population contains a small proportion of about 12% of cells expressing Gr-1, CD11b, and CD11c, indicating that our specific MDSC culturing conditions might not be causative responsible for the appearance of the CD11b+CD11c+Gr-1+ MDSC subpopulation.
In conclusion, we show that MDSCs might be useful as cellular therapy in GVHD prevention because their ability to skew the immune response toward type 2 immunity abrogates GVHD while maintaining tumor cytotoxicity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maxi Weiswange, Sylvia Muche, and Sarah Gehring for excellent technical assistance.
This study was supported by the Deutsche José Carreras Leukämiestiftung e.V. (DJCLS R10/06).
Authorship
Contribution: J.J.M. performed experiments and analyzed data; T.R. performed intracellular stainings and homing experiments; F.L. performed histologic analysis and reviewed the data; M.B.L. gave technical advice and edited the manuscript; K.-M.D. contributed to the experimental design and edited the manuscript; and G.S. conceived experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gudrun Strauss, University Medical Center Ulm, Department of Pediatrics and Adolescent Medicine, Eythstr. 24, 89075 Ulm, Germany; e-mail: gudrun.strauss@uniklinik-ulm.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal