Key Points
Vaccination with HBZ can induce cytotoxic T lymphocytes and suppress an HBZ-expressing lymphoma cell line in vivo.
Immunodominant epitopes of HBZ have been identified in mice, monkeys, and humans.
Abstract
Human T-cell leukemia virus type 1 (HTLV-1) causes adult T-cell leukemia-lymphoma (ATL) and inflammatory diseases in a small percentage of infected individuals. Host immune responses, in particular cytotoxic T lymphocytes (CTLs), influence the proliferation and survival of ATL cells and HTLV-1–infected cells. We generated recombinant vaccinia viruses (rVVs) expressing HTLV-1 basic leucine zipper (bZIP) factor (HBZ) or Tax to study the immunogenic potential of these viral proteins. Vaccination with rVV expressing Tax or HBZ induced specific T-cell responses, although multiple boosters were needed for HBZ. HBZ-stimulated T cells killed HBZ peptide-pulsed T cells and CD4+ T cells from HBZ transgenic (HBZ-Tg) mice. The anti-lymphoma effect of the CTLs targeting HBZ was tested in mice inoculated with a lymphoma cell line derived from an HBZ-Tg mouse. Transfer of splenocytes from HBZ-immunized mice increased the survival of the lymphoma cell–inoculated mice, suggesting that the anti-HBZ CTLs have a protective effect. The rVV could also induce specific T-cell responses to HBZ and Tax in HTLV-1–infected rhesus monkeys. On the basis of the results of rVV-vaccinated mice and macaques, we identified a candidate peptide (HBZ157-176) for vaccine development. Dendritic cells pulsed with this peptide could generate HBZ-specific CTLs from human CD8+ T cells. This study demonstrates that HBZ could be a target for immunotherapy of patients with ATL.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) infects primarily peripheral CD4+ T cells in vivo and causes a malignant disease, adult T-cell leukemia-lymphoma (ATL) and inflammatory diseases that include HTLV-1 associated myelopathy/tropical spastic paraparesis, bronchitis, and uveitis.1-3 HTLV-1 infects cells mainly by cell-to-cell transmission.4 This virus spreads in vivo by two routes: de novo infection of cells (infectious spread) and proliferation of infected cells (mitotic spread).5-7 It has been reported that reverse transcriptase inhibitors and integrase inhibitors do not affect the proviral load in HTLV-1–infected individuals,8,9 indicating that mitotic spread is predominant in the chronic phase of HTLV-1 infection. Proliferation of infected cells is promoted by the expression of viral genes, in particular, the tax and HTLV-1 basic leucine zipper (bZIP) factor (HBZ) genes.3,10 Concurrently, HTLV-1–infected cells are suppressed by host immune responses, especially cytotoxic T lymphocytes (CTLs).11
HTLV-1 encodes several regulatory and accessory genes in the viral genome.12 Tax, which is encoded in the plus strand of the provirus, strongly induces the proliferation of infected cells via the transactivation of nuclear factor kappa B (NF-κB) and activating protein-1.13 Recently, Tax-induced NF-κB hyperactivation has been reported to cause cellular senescence in Hela cells,14 suggesting that Tax expression may be a disadvantage for the survival of infected cells. Furthermore, Tax is an immunodominant antigen of this virus and is targeted by CTLs.11 Therefore, Tax expression tends to be suppressed during the long latent period of the virus. In contrast, HBZ, which is encoded in the minus strand, is constitutively expressed in infected cells15 and promotes proliferation of T cells in vivo and in vitro.15 Importantly, transgenic expression of HBZ has been reported to cause T-cell lymphomas and inflammatory diseases.16 HBZ is also known to perturb cell signaling and cytokine production in ways that lead to immunodeficiency and inflammation.17-19
Tax expression is frequently lost during the leukemic stage of HTLV-1 infection, whereas HBZ is constantly expressed,15,20,21 suggesting that HBZ is a potentially useful target for vaccine treatment. On the basis of a mathematical model, Macnamara et al22 have shown that it is the immune response against HBZ, rather than that against Tax, that influences the proviral load. The presence and induction of T-cell or B-cell memory responses against HBZ have been reported in vivo and in vitro,23-26 suggesting that HBZ could be a candidate antigen for vaccine therapy.
In this study, we induced HBZ-specific T-cell responses in mice by using recombinant vaccinia virus (rVV). In addition, a T-cell lymphoma cell line was established from HBZ-expressing transgenic mice (HBZ-Tg). The rVV-induced specific memory response improved the survival of HBZ-induced lymphoma-challenged mice. Finally, we identified potential peptides for an HBZ vaccine by using rVV-vaccinated mice and monkeys.
Methods
Animals and patients
All animal experiments that used mice and monkeys were approved by Kyoto University (approval numbers D12-02, D13-02, D14-02, R12-01, R13-01, and R14-01). C57BL/6 (Ly5.2), nonobese diabetic-severe combined immunodeficiency (NOD-SCID ), common γ-chain knockout (NOG, and SCID mice were purchased from the Central Institute for Experimental Animals (Kawasaki, Japan) and CLEA Japan (Tokyo, Japan). Ly5.1 C57BL/6 mice were a gift from A. Shimizu (Kyoto University Hospital). Ly5.1 C57BL/6 mice were used to discriminate effector and target T cells. Transgenic mice expressing the spliced form of the HBZ gene under the control of the CD4 promoter/enhancer/silencer have been described previously.15 All mice (6-14 weeks of age) used in this study were maintained in a specific pathogen free facility. Blood samples were collected from the ATL patients, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque Plus (GE Healthcare Bio-Sciences) density gradient centrifugation. This study was conducted according to the principles in the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Kyoto University (approval number G310).
Generation of rVV and vaccination protocol
All rVVs used in this experiment were generated as reported previously.27 In brief, the antigen’s gene was inserted into the LC16m8 strain of VV in place of the hemagglutinin gene by homologous recombination with pBMSF7c plasmid in chicken embryonic fibroblasts. An NF-κB transactivation-deficient mutant of HTLV-1 Tax, M22 (T130A/L131S), which still activates the cAMP response element binding protein (CREB)/activating transcription factor (ATF) pathway,28 and an HBZ-LL/AA (L27A/L28A) mutant, which cannot activate the transforming growth factor beta/Smad pathway,29 were used as antigens. The rVVs generated were cloned by adsorption with chicken red blood cells on RK13 cells. Purified rVVs were propagated and titrated on the RK13 cell line and stored at −80°C until use. The expression of the gene inserted in rVV was evaluated by immunoblotting. MI73 antibody was used for the detection of Tax antigen.21 HBZ expression was detected with a rabbit serum obtained from animals immunized with HBZ peptides (CRGPPGEKAPPRGETH and QERRERKWRQGAEKC) (Medical Biological Laboratories).
Inoculations were performed by skin scarification in mice (C57BL/6) and monkeys after shaving the area. Each animal received a dose of 107 plaque-forming units of rVV in 10 μL of viral suspension. In mice, 4 weeks after the first vaccination, 5 booster vaccinations were administered every 3 weeks. To induce an effective immune reaction, scarification sites were changed every time. Splenocytes were isolated 1 week after the last inoculation and stored in liquid nitrogen until the assay was performed. In monkeys, booster vaccinations were repeated every 4 weeks. PBMCs from monkeys were obtained every 2 weeks.
Establishment and characterization of the Ht48 cell line
An HBZ-expressing mouse T-cell line, Ht48, was established by the serial transplantation of lymphoma cells from an HBZ-Tg mouse into immunodeficient mice. Briefly, isolated cells were first inoculated intraperitoneally into NOG mice. When mice started showing signs of wasting, spleens were taken. Bulk splenocytes were injected into SCID mice. The same procedure was followed to eventually transfer cells to wild-type (WT) C57BL/6 mice, resulting in development of lymphoma. At this point, Ht48 cells could be maintained in vivo by serial transplantation using WT C57BL/6 mice. Tissue samples from Ht48-inoculated C57BL/6 mice were fixed in 10% formalin in phosphate buffer and embedded in paraffin. Hematoxylin and eosin staining was performed according to standard protocols. For cytotoxicity assays, Ht48 cells were cultured in vitro for 4 weeks in RPMI 1640 supplemented by recombinant interleukin-2 (IL-2) and 5.5 μM 2-mercaptoethanol (Gibco). Thereafter, this cell line was cultured in the absence of IL-2.
HTLV-1 infection of rhesus macaques
To establish an HTLV-1 carrier monkey model, two 7-year-old rhesus macaques (Macaca mulatta: MM557 and MM558) were anesthetized and intraperitoneally inoculated with 5 × 107 viable MT-2 cells. HTLV-1 infection in these monkeys was confirmed by the detection of anti-HTLV-1 antibodies using the SERODIA-HTLV-1 kit (Fuji Rebio), which used a particle agglutination method.
Peptide reconstitution assay
HTLV-1–infected cell lines (TL-Om1 and MT-2) were washed once with RPMI medium and then treated with acid solution (0.131 M citric acid/0.066M Na2HPO4 [pH3.0]) for 90 seconds. Then, 5 × 105 cells were incubated in the presence of β2-microglobulin (1 mg/mL) and 10 mM peptide for 4 hours at 29°C. HLA reconstitution was evaluated by staining with anti-HLA–A2 and –A24 monoclonal antibody and using flow cytometry. Percent mean fluorescent intensity (MFI) increase = [(HLA-MFI with peptide – HLA-MFI without peptide)/HLA-MFI without peptide] × 100.
Induction of human CTLs against HBZ
HLA expression in PBMCs from a healthy donor was confirmed by staining with antibodies to HLA-A2 (BB7.2) and HLA-A24 (17A10) and was analyzed by flow cytometry. For dendritic cell (DC) induction, donor CD14+ cells were enriched from PBMCs by using magnetic particles (BD Bioscience) and cultured in AIM-V medium (Gibco) supplemented with 4% donor plasma, 10 ng/mL human granulocyte macrophage colony-stimulating factor, and 10 ng/mL human IL-4 (Miltenyi Biotec). After 6 days of induction, cells were stimulated with 0.1 klinische einheit/mL of OK-432 (Chugai Pharmaceutical Co.) for 48 hours. Induced monocyte-derived DCs were pulsed with 10 μM peptides and irradiated via radiographs. Then, 105 DCs were cocultured with 106 donor CD8+ T cells in the presence of 1 μg/mL human IL-7 (Miltenyi Biotec) for 6 days. IL-2 was added at 20 U/mL after 48 hours. The presensitization was done 3 times, and the specific reaction of CTLs was measured by cytokine production after stimulation with peptide-pulsed auto-B-lymphoblastoid cell lines (B-LCLs).
Statistical analysis
Statistical analyses of in vitro experiments or mouse survival were performed by using the Student unpaired t test or log-rank test, respectively.
Results
Vaccination with rVV-expressing HTLV-1 antigens elicits a T-cell response
We generated rVV vaccines expressing mutated HTLV-1 proteins that lacked important functions of these proteins (the Tax-M22 and HBZ-LL/AA mutants). Tax-M22 cannot activate the NF-κB pathway,28 and HBZ-LL/AA lacks the capacity to activate the transforming growth factor beta/Smad pathway.29 Infection with rVV-Tax-M22 and HBZ-LL/AA induced expression of the inserted antigens in RK13 cells (supplemental Figure 1A-B available at the Blood Web site). First, we inoculated 107 plaque-forming units of rVV expressing HBZ-LL/AA or Tax-M22 into mice to induce specific immune responses. The number of interferon-γ (IFN-γ) –producing cells was quite low after a single inoculation with HBZ-LL/AA, whereas a single inoculation with rVV-Tax-M22 induced a strong CTL response (Figure 1A). However, after inoculation 6 times with rVV-HBZ-LL/AA, an enzyme-linked immunospot (ELISPOT) assay detected CD4+ and CD8+ T cells that produced IFN-γ in response to pooled 6mer-overlapping HBZ 20mers (especially HBZ-P2, overlapping peptides spanning the 49-104 region [HBZ49-104]; HBZ-P3, overlapping peptides spanning the 91-152 region [HBZ91-152]; and HBZ-P4, overlapping peptides spanning the 139-206 region [HBZ139-206]; but not HBZ-P1: overlapping peptides spanning the 49-104 region [HBZ1-92]) (Figure 1B-C). Tax-specific T-cell responses were also detected (Figure 1B-C).
Induction of HTLV-1 antigen-specific T cells by rVV in mice. (A) Mice (n = 3) in each group received a single rVV vaccination. Whole splenocytes were pooled and IFN-γ production was measured after stimulation (stimu) with overlapping peptides. (B) Mice were vaccinated with the rVV as shown. IFN-γ–producing cells among CD4- or CD8-depleted splenocytes were measured after stimulation with the pooled peptides (P1-P4). Data are presented as total number of spots from 4 measured pools. The bars represent median values. (C) The proportion of T cells isolated from rVV Tax M22- or HBZ LL/AA-vaccinated mice reacting to each peptide pool. The results shown are the average for each pool. (D) CD8+ T cells from vaccinated mice were stimulated as indicated, and cells positive for IFN-γ, CD107a, TNF-α, or IL-2 were measured. Cells stimulated with nonpulsed EL4 cells were used as a reference. The bars represent the mean ± standard deviation (SD) for all mice. At least 2 independent experiments were performed. *P < .05 by Student t test. APC, antigen-presenting cell.
Induction of HTLV-1 antigen-specific T cells by rVV in mice. (A) Mice (n = 3) in each group received a single rVV vaccination. Whole splenocytes were pooled and IFN-γ production was measured after stimulation (stimu) with overlapping peptides. (B) Mice were vaccinated with the rVV as shown. IFN-γ–producing cells among CD4- or CD8-depleted splenocytes were measured after stimulation with the pooled peptides (P1-P4). Data are presented as total number of spots from 4 measured pools. The bars represent median values. (C) The proportion of T cells isolated from rVV Tax M22- or HBZ LL/AA-vaccinated mice reacting to each peptide pool. The results shown are the average for each pool. (D) CD8+ T cells from vaccinated mice were stimulated as indicated, and cells positive for IFN-γ, CD107a, TNF-α, or IL-2 were measured. Cells stimulated with nonpulsed EL4 cells were used as a reference. The bars represent the mean ± standard deviation (SD) for all mice. At least 2 independent experiments were performed. *P < .05 by Student t test. APC, antigen-presenting cell.
It has been reported that double/triple cytokine (IFN-γ/tumor necrosis factor α [TNF-α]/IL-2) –producing CTLs are more functional than single cytokine-producing CTLs.30 To investigate specific CTL function, CD8+ T cells were isolated from vaccinated mice and stimulated as shown in Figure 1D. Overlapping peptides were pooled as Tax-PA (Tax1-182) and Tax-PB (Tax169-353) for Tax and HBZ-PA (HBZ1-110) and HBZ-PB (HBZ97-206) for HBZ. The number of HBZ- or Tax-specific CD8+ T cells that were IFN-γ, TNF-α, or CD107a positive was increased in vaccinated mice compared with WT VV-inoculated mice. However, IL-2 production was increased only in Tax-specific CTLs (Figure 1D).
In CD4+ T cells, the production of most cytokines was enhanced (IL-2, IL-4, IL-5, IL-6, TNF-α) in Tax-immunized mice (supplemental Figure 2). However, in HBZ-immunized mice, only IL-2 production was slightly increased, indicating that the helper function induced by HBZ immunization is weak compared with that induced by Tax. When we checked the VV antigen-specific T-cell response as an internal control, there were no significant differences between mice vaccinated with WT-VV, rVV-Tax-M22, or rVV-HBZ-LL/AA (supplemental Figure 3A-C). These data indicate that the immunogenicity of HBZ is lower than that of Tax. However, repetitive immunization induced a T-cell response to HBZ.
rVV-HBZ-LL/AA confers protective immunity in mice
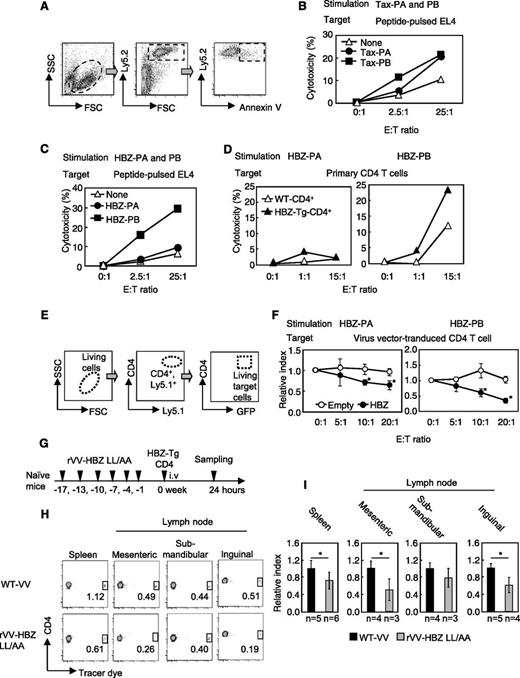
To further investigate the function of HBZ-specific CTLs, we performed in vivo and in vitro cytotoxicity assays. By using annexin V staining, we measured their ability to kill peptide-pulsed EL4 cells, as shown in Figure 2A. First, as an internal control, we measured specific cytotoxicity against a VV peptide. Cytotoxic activity in response to VV was observed in effector cells from both the rVV-Tax-M22– and rVV-HBZ-LL/AA–vaccinated mice (supplemental Figure 3D), indicating that vaccination induced CTLs to VV. Likewise, effector cells from rVV-Tax-M22–immunized mice attacked both Tax-PA– and Tax-PB–pulsed mouse T-cell line EL4 cells (Figure 2B). Conversely, HBZ effector cells killed the EL4 cells pulsed with HBZ-PB but not HBZ-PA peptides (Figure 2C). In accordance with this finding, only HBZ-PB–stimulated effector cells showed enhanced cytotoxicity against HBZ-Tg CD4+ T cells (Figure 2D), suggesting that HBZ-PB contains the major epitopes for CTLs.
rVV-induced effector cells from vaccinated mice kill CD4+ T cells from HBZ-Tg mice. (A) Schema of the cytotoxicity assay using flow cytometry. Ly5.2 target cells were cocultured with Ly5.1 effector cells. Annexin V– and Ly5.2-positive cells were measured to evaluate cytotoxicity. (B) Cytotoxicity of effector cells from rVV-Tax M22–vaccinated mice. (C) HBZ-PA/HBZ-PB–pulsed EL-4 cells or (D) primary Ly5.2+CD4+ T cells from HBZ-Tg mice were cocultured with Ly5.1+ HBZ effector cells, and cytotoxicity against target cells was measured. Ly5.1 and Ly5.2 were used as markers to discriminate effector T cells and target T cells. (E-F) Cytotoxicity of HBZ-specific effector cells against HBZ-overexpressing CD4+ T cells. (E) Primary Ly5.1+CD4+ T cells were transduced with HBZ and used as target cells in coculture with Ly5.2+ HBZ effector cells. The GFP+ target cells among Ly5.1+ and CD4+ cells were measured. (F) Relative index = E:T(x:1)/E:T(0:1) in GFP-positive cells (%). (G-I) In vivo cytotoxicity assay. (G) Naïve mice were vaccinated 6 times and inoculated with Tracer dye–labeled HBZ-Tg CD4+ T cells; 24 hours later, the percentage of labeled cells was measured in the CD4+ population. (H-I) Transferred HBZ-Tg CD4+ T cells in spleen and mesenteric, submandibular, and inguinal lymph nodes of recipient mice. The percentage of tracer dye–positive cells is shown in the dot plots. The data from WT VV-vaccinated mice was used as a reference. The bars represent the mean ± SD of all mice. At least 2 independent experiments were performed. *P < .05 by Student t test. i.v., intravenous. SSC, side scatter; FSC, forward scatter; E:T, effector:target; GFP, green fluorescent protein.
rVV-induced effector cells from vaccinated mice kill CD4+ T cells from HBZ-Tg mice. (A) Schema of the cytotoxicity assay using flow cytometry. Ly5.2 target cells were cocultured with Ly5.1 effector cells. Annexin V– and Ly5.2-positive cells were measured to evaluate cytotoxicity. (B) Cytotoxicity of effector cells from rVV-Tax M22–vaccinated mice. (C) HBZ-PA/HBZ-PB–pulsed EL-4 cells or (D) primary Ly5.2+CD4+ T cells from HBZ-Tg mice were cocultured with Ly5.1+ HBZ effector cells, and cytotoxicity against target cells was measured. Ly5.1 and Ly5.2 were used as markers to discriminate effector T cells and target T cells. (E-F) Cytotoxicity of HBZ-specific effector cells against HBZ-overexpressing CD4+ T cells. (E) Primary Ly5.1+CD4+ T cells were transduced with HBZ and used as target cells in coculture with Ly5.2+ HBZ effector cells. The GFP+ target cells among Ly5.1+ and CD4+ cells were measured. (F) Relative index = E:T(x:1)/E:T(0:1) in GFP-positive cells (%). (G-I) In vivo cytotoxicity assay. (G) Naïve mice were vaccinated 6 times and inoculated with Tracer dye–labeled HBZ-Tg CD4+ T cells; 24 hours later, the percentage of labeled cells was measured in the CD4+ population. (H-I) Transferred HBZ-Tg CD4+ T cells in spleen and mesenteric, submandibular, and inguinal lymph nodes of recipient mice. The percentage of tracer dye–positive cells is shown in the dot plots. The data from WT VV-vaccinated mice was used as a reference. The bars represent the mean ± SD of all mice. At least 2 independent experiments were performed. *P < .05 by Student t test. i.v., intravenous. SSC, side scatter; FSC, forward scatter; E:T, effector:target; GFP, green fluorescent protein.
To assess cytotoxicity against endogenous HBZ-expressing cells, we cocultured Ly5.2-effector cells (generated as shown in Figure 2C) with Ly5.1-CD4+ HBZ-transduced target cells (obtained as shown in Figure 2E). Compared with control CD4+ T cells, HBZ-expressing CD4+ T-cell targets were suppressed in the presence of HBZ-PA and HBZ-PB effector cells (Figure 2F).
Next, we evaluated the cytotoxicity of T cells in vivo. CD4+ T cells of HBZ-Tg mice were transferred into vaccinated mice, and 24 hours later, the number of viable transferred CD4+ T cells was measured (Figure 2G). Transferred cells were significantly reduced in the spleen and the mesenteric and inguinal lymph nodes of rVV-HBZ-LL/AA–vaccinated mice (Figure 2H-I), indicating that rVV-HBZ-LL/AA induces a cytotoxic memory response against HBZ-expressing CD4+ T cells in vivo.
Establishment of an HBZ-induced ATL model and protection by the HBZ-specific T-cell response
To evaluate cytotoxicity against HBZ-expressing lymphoma cells, we established an HBZ-induced T-cell line from a lymphoma that developed spontaneously in HBZ-Tg mice. Cells isolated from the enlarged lymph node of an HBZ-Tg mouse were injected into immunodeficient mice as described in “Methods.” Subsequently, this cell line was maintained in vivo, and it finally formed tumors in inoculated C57BL/6 mice. This cell line was CD4+CD8+CD25+ at first, but it lost CD25 expression during the subsequent transfers (Figure 3A). In addition, expression of both CD4 and CD8 was gradually decreased during passages (Figure 3A). The cell line was designated Ht48. Ht48 cells, which express the HBZ gene at a level similar to that of CD4+ T cells from HBZ-Tg and primary ATL cells (Figure 3B), killed inoculated mice in a dose-dependent manner (Figure 3C), and tended to infiltrate in liver and lymphoid tissues in vivo (Figure 3D-E). In the Ht48 cell line, we observed expression of T-cell receptor β chain and V(D)J recombination of T-cell receptor beta, confirming that Ht48 is a T-cell line (supplemental Figure 6).
Establishment of an HBZ-induced T-cell line. (A) Expression of various markers in the Ht48 cell line. Cells isolated from the lymph nodes (LN) of an HBZ-Tg mouse were injected intraperitoneally into immunodeficient (NOG and SCID) and immunocompetent mice (C57BL/6) as indicated. Eventually, splenocytes from a C57BL/6 mouse established the cell line we named Ht48. In vivo passage of this cell line was done by serial transplantation. (B) HBZ messenger RNA (mRNA) level in the Ht48 cell line. (C) Intraperitoneal inoculation of Ht48 into C57BL/6 mice decreased their survival in a dose-dependent manner. Survival curves were analyzed by the Kaplan-Meier method, and statistical difference was calculated by log-rank test. *P < .05 by log-rank test. (D) Ht48 inoculation (105 cells) via tail vein resulted in severe accumulation of cells in the liver and spleen after 4 weeks of inoculation. (E) Hematoxylin and eosin staining of tissues from the Ht48-inoculated mice. The data shown represent the mean ± SD of triplicate experiments. At least 2 independent experiments were performed. p.i., post inoculation.
Establishment of an HBZ-induced T-cell line. (A) Expression of various markers in the Ht48 cell line. Cells isolated from the lymph nodes (LN) of an HBZ-Tg mouse were injected intraperitoneally into immunodeficient (NOG and SCID) and immunocompetent mice (C57BL/6) as indicated. Eventually, splenocytes from a C57BL/6 mouse established the cell line we named Ht48. In vivo passage of this cell line was done by serial transplantation. (B) HBZ messenger RNA (mRNA) level in the Ht48 cell line. (C) Intraperitoneal inoculation of Ht48 into C57BL/6 mice decreased their survival in a dose-dependent manner. Survival curves were analyzed by the Kaplan-Meier method, and statistical difference was calculated by log-rank test. *P < .05 by log-rank test. (D) Ht48 inoculation (105 cells) via tail vein resulted in severe accumulation of cells in the liver and spleen after 4 weeks of inoculation. (E) Hematoxylin and eosin staining of tissues from the Ht48-inoculated mice. The data shown represent the mean ± SD of triplicate experiments. At least 2 independent experiments were performed. p.i., post inoculation.
We observed that HBZ-PA– or HBZ-PB–stimulated effector cells showed enhanced cytotoxicity against Ht48 cells compared with EL4 cells (Figure 4A). An in vivo survival assay was performed as shown in Figure 4B. First, Ht48 cells were inoculated intraperitoneally into naïve WT mice. Subsequently, splenocytes from rVV-HBZ-LL/AA–immunized mice were transferred 3 times via the same route. This partially rescued Ht48-inoculated mice compared with mice that received transfer of splenocytes from WT-VV or no transfer (Figure 4B), indicating that the rVV-HBZ-LL/AA–stimulated T-cell response could suppress Ht48 cells in vivo.
Adoptive transfer of rVV-induced CTLs improves survival in an HBZ-induced ATL mouse model. (A) The cytotoxic effect of HBZ effector cells (Ly5.1) against Ht48 cells (Ly5.2). EL4 cells were used as a negative control. (B) HBZ-specific effector cells improved the survival ratio in an HBZ-induced ATL model. Naïve mice were inoculated intraperitoneally (i.p.), first with Ht48 cells, and then 3 times with peptide-stimulated splenocytes from rVV-HBZ LL/AA–vaccinated mice. At least 2 independent experiments were performed. Survival curves were analyzed by the Kaplan-Meier method, and statistical difference was calculated by log-rank test. *P < .05 by log-rank test.
Adoptive transfer of rVV-induced CTLs improves survival in an HBZ-induced ATL mouse model. (A) The cytotoxic effect of HBZ effector cells (Ly5.1) against Ht48 cells (Ly5.2). EL4 cells were used as a negative control. (B) HBZ-specific effector cells improved the survival ratio in an HBZ-induced ATL model. Naïve mice were inoculated intraperitoneally (i.p.), first with Ht48 cells, and then 3 times with peptide-stimulated splenocytes from rVV-HBZ LL/AA–vaccinated mice. At least 2 independent experiments were performed. Survival curves were analyzed by the Kaplan-Meier method, and statistical difference was calculated by log-rank test. *P < .05 by log-rank test.
rVVs induce memory T-cell responses in HTLV-1–infected rhesus monkeys
To analyze immune responses to HTLV-1 in nonhuman primates, we infected 2 rhesus macaques (MM557 and MM558) with HTLV-1 by inoculating them with nonirradiated MT-2 cells. HTLV-1 infection was confirmed by the presence of anti-HTLV-1 antibodies in the plasma (supplemental Figure 4). However, proviral load was quite low in both monkeys (MM557, 0.1%; MM558, less than 0.1%), and CTLs were not detected in either monkey by ELISPOT assay. Therefore, to induce a CTL response in these monkeys, we vaccinated MM557 with rVV-Tax-M22 and MM558 with rVV-HBZ-LL/AA. Vaccinating MM557 with rVV-Tax-M22 produced strong responses of IFN-γ−producing CD4+ and CD8+ T cells (Figure 5A). Although HBZ-specific T cells in vaccinated MM558 were detected after 8 weeks of vaccination (Figure 5B), the number of spots in MM558 was lower than that in MM557, suggesting that the immunogenicity of HBZ antigen is low in monkeys as well as in mice. PBMCs from vaccinated monkeys were stimulated as shown in Figure 5C, and cytokine production was measured. MM557 PBMCs stimulated by Tax-PA or Tax-PB showed increased IFN-γ and TNF-α production by CD8+ T cells (Figure 5D). In contrast, whereas HBZ-PB-stimulated MM558 PBMCs produced both cytokines, HBZ-PA stimulation induced only TNF-α production (Figure 5E). These results were consistent with those obtained with stimulated mouse cells, indicating a lower immune response to HBZ-PA than to HBZ-PB. These data show that immune responses to Tax and HBZ can be potentiated by vaccination.
Induction of specific T-cell responses in HTLV-1–infected rhesus monkeys (Macaca mulatta) by rVVs. (A-B) The antigen-specific T-cell response in the monkeys designated (A) MM557 and (B) MM558 was measured in CD4- or CD8-depleted PBMCs by IFN-γ ELISPOT assay. Cells were stimulated with Tax or HBZ pooled peptides (P1-P4). (C-E) IFN-γ and TNF-α production by Tax- or HBZ-specific CD8+ T cells induced by (C) rVV. For IFN-γ and TNF-α production, PBMCs from (D) MM557 and (E) MM558 were isolated 39 weeks after vaccination. Cells were preactivated with pooled peptides and restimulated with peptide-pulsed auto PBMCs, which were labeled with a tracer dye to discriminate between responder and stimulator cells. Cytokine production in CD8+ T cells was measured in the tracer dye–negative population. The percentage of cytokine-producing cells is shown in the dot plots. Phorbol myristate acetate (PMA) and ionomycin (P/I) were used as a positive control of the stimulus.
Induction of specific T-cell responses in HTLV-1–infected rhesus monkeys (Macaca mulatta) by rVVs. (A-B) The antigen-specific T-cell response in the monkeys designated (A) MM557 and (B) MM558 was measured in CD4- or CD8-depleted PBMCs by IFN-γ ELISPOT assay. Cells were stimulated with Tax or HBZ pooled peptides (P1-P4). (C-E) IFN-γ and TNF-α production by Tax- or HBZ-specific CD8+ T cells induced by (C) rVV. For IFN-γ and TNF-α production, PBMCs from (D) MM557 and (E) MM558 were isolated 39 weeks after vaccination. Cells were preactivated with pooled peptides and restimulated with peptide-pulsed auto PBMCs, which were labeled with a tracer dye to discriminate between responder and stimulator cells. Cytokine production in CD8+ T cells was measured in the tracer dye–negative population. The percentage of cytokine-producing cells is shown in the dot plots. Phorbol myristate acetate (PMA) and ionomycin (P/I) were used as a positive control of the stimulus.
The peptide sequence responsible for HBZ-specific CD8+ T cells
Previous studies using algorithmic analysis and clinical samples have suggested a candidate peptide antigen for HBZ-specific T cells.22,23,25 We identified candidate peptides in our system, peptides that activate memory T cells from rVV-HBZ-LL/AA–vaccinated mice and monkeys. Splenocytes isolated from vaccinated mice or PBMCs isolated from vaccinated monkeys were prestimulated with HBZ-PA or HBZ-PB. Because HBZ-P1 did not induce a T-cell response when stimulated with pooled peptides (Figure 1C), the cells were restimulated with bone marrow–derived DC that had been pulsed with a matched single peptide from three pools (HBZ-P2, -P3, and -P4). Candidate peptides that were able to activate prestimulated CD8+ T cells from vaccinated mice were HBZ61-86, HBZ109-128, HBZ133-152, HBZ145-164, HBZ157-176, and HBZ187-206. Conversely, monkey CD8+ T cells were activated by HBZ109-128, HBZ127-146, HBZ133-152, HBZ145-164, HBZ157-176, and HBZ187-206 (Figure 6A-B). We focused on HBZ109-128 and HBZ157-176 because of the strong T-cell responses they elicited in mice and monkeys. To evaluate whether HBZ-expressing cells were killed by effector cells stimulated with these two peptides, we performed a cytotoxicity assay. Target cells were prepared by transfecting an HBZ expression plasmid to auto-B-LCL (Figure 6C). Whereas HBZ109-128-stimulated PBMCs had no killing activity, HBZ157-176-stimulated effector cells reduced the population of cocultured target cells in a concentration-dependent manner (Figure 6D). Next, we used this peptide to stimulate human T cells (HLA-A2/HLA-A24–positive healthy donor). HBZ157-176-pulsed DCs induced specific CTLs in response to matched peptide stimulation in a peptide-dependent manner (Figure 6E-G). Furthermore, peptide reconstitution assay showed that the candidate peptides bound to HLA-A2 and HLA-A24 (Figure 6H). This peptide could also induce an HBZ-specific T-cell response in mouse splenocytes when mice were subcutaneously immunized with HBZ157-176 and cytosine phosphate guanine as an adjuvant (supplemental Figure 5A). In addition, CD4+ T cells from vaccinated mice also reacted to HBZ157-176 (supplemental Figure 5B). From these results, we identified HBZ157-176 as a candidate for an HBZ peptide vaccine.
HBZ157-176 is a candidate for the development of an anti-HTLV-1 peptide vaccine. (A-B) Mouse splenocytes and MM558 PBMCs were prestimulated with (A) HBZ-PA or (B) HBZ-PB. Thereafter, mouse (upper panels) and monkey (lower panels) cells were restimulated with single peptide–pulsed DCs (for mice) or single peptide–pulsed auto-PBMCs (for monkeys). IFN-γ and TNF-α production in CD8+ T cells were measured. Cells stimulated with nonpulsed APCs were used as a reference. (C-D) Cytotoxicity assay using B-lymphoblastoid cell lines (B-LCL) overexpressing HBZ. (C) B-LCLs from MM558 were transfected with HBZ, labeled with tracer dye, and used as target cells. (D) Target cells were cocultured with HBZ109-128- or HBZ157-176-stimulated MM558 PBMCs, and cytotoxicity was evaluated by counting living target cells. Relative index = E:T(x:1)/E:T(0:1) in GFP-positive cells (%). (E-G) Cytokine production by HBZ-specific CTLs. (E) HLA-A2 and HLA-A24 expression in PBMCs from a healthy donor. (F) Production of IFN-γ and TNF-α. CTLs were induced by using HBZ109-128 or HBZ157-176-pulsed DCs from an HLA-A2– and HLA-A24–positive healthy donor. The cells were cocultured with auto-B-LCLs pulsed with the matched peptide, and production of IFN-γ and TNF-α was analyzed. (G) HBZ-specific CTLs generated by HBZ157-176-pulsed DCs produced cytokines in a concentration-dependent manner. (H) Peptide reconstitution assay of HBZ109-128 and HBZ157-176 peptide on HLA-positive HTLV-1–infected and ATL cell lines. Binding of HLA and the peptides was measured by using HTLV-1–infected cell lines TL-Om1 and MT-2. OVA323-339 peptide was used as negative control. Peptide-HLA binding was measured as a recovered mean fluorescence intensity (MFI) of HLA-A2 and HLA-A24 by using flow cytometry. The bars represent the mean ± SD of triplicate experiments.
HBZ157-176 is a candidate for the development of an anti-HTLV-1 peptide vaccine. (A-B) Mouse splenocytes and MM558 PBMCs were prestimulated with (A) HBZ-PA or (B) HBZ-PB. Thereafter, mouse (upper panels) and monkey (lower panels) cells were restimulated with single peptide–pulsed DCs (for mice) or single peptide–pulsed auto-PBMCs (for monkeys). IFN-γ and TNF-α production in CD8+ T cells were measured. Cells stimulated with nonpulsed APCs were used as a reference. (C-D) Cytotoxicity assay using B-lymphoblastoid cell lines (B-LCL) overexpressing HBZ. (C) B-LCLs from MM558 were transfected with HBZ, labeled with tracer dye, and used as target cells. (D) Target cells were cocultured with HBZ109-128- or HBZ157-176-stimulated MM558 PBMCs, and cytotoxicity was evaluated by counting living target cells. Relative index = E:T(x:1)/E:T(0:1) in GFP-positive cells (%). (E-G) Cytokine production by HBZ-specific CTLs. (E) HLA-A2 and HLA-A24 expression in PBMCs from a healthy donor. (F) Production of IFN-γ and TNF-α. CTLs were induced by using HBZ109-128 or HBZ157-176-pulsed DCs from an HLA-A2– and HLA-A24–positive healthy donor. The cells were cocultured with auto-B-LCLs pulsed with the matched peptide, and production of IFN-γ and TNF-α was analyzed. (G) HBZ-specific CTLs generated by HBZ157-176-pulsed DCs produced cytokines in a concentration-dependent manner. (H) Peptide reconstitution assay of HBZ109-128 and HBZ157-176 peptide on HLA-positive HTLV-1–infected and ATL cell lines. Binding of HLA and the peptides was measured by using HTLV-1–infected cell lines TL-Om1 and MT-2. OVA323-339 peptide was used as negative control. Peptide-HLA binding was measured as a recovered mean fluorescence intensity (MFI) of HLA-A2 and HLA-A24 by using flow cytometry. The bars represent the mean ± SD of triplicate experiments.
Discussion
Many viruses that cause chronic infections, including hepatitis C virus, human immunodeficiency virus, Epstein-Barr virus, herpes simplex virus, and HTLV-1, have strategies for evading the host immune response.31,32 It is especially difficult to eliminate the virus in its latent period, when only viral nonstructural proteins (VNPs) are expressed. The immunogenicity of many VNPs is lower than that of viral structural proteins. The host immune system effectively eliminates the invading virus during acute infection when it expresses viral structural proteins. VNPs play a key role in the malignant transformation of infected cells and in mechanisms that help the virus escape from the host immune system.31 For HTLV-1, it has been reported that vaccines expressing the envelope protein completely block infection in animal models.33,34 Among HTLV-1 regulatory and accessory proteins, Tax is known to be the immunodominant protein, and a Tax vaccine was reported to protect against HTLV-1 infection in an animal model.35 However, because of the high immunogenicity of Tax, its expression is frequently silenced or lost in ATL patients to evade the host immune surveillance.21
Conversely, there are no reports on vaccine development for HBZ. In contrast to Tax, HBZ expression is maintained in ATL cells, indicating that HBZ could serve as a potential target for immunotherapy in ATL. However, although the presence of anti-HBZ CTLs has been reported, it remains unknown whether anti-HBZ CTLs can be induced in vivo.23,36 In this study, we show that rVV-expressing HBZ can induce specific CTL responses, and vaccination with HBZ partially protects mice from HBZ-expressing T-cell lymphoma. Furthermore, HBZ157-176 was identified as a candidate sequence for the development of a peptide-based vaccine. Longer peptides containing multiple epitopes for T cells have been known to induce stronger T-cell responses than shorter peptides.37 It is well known that epitopes for CD8+ T cells presented on major histocompatibility complex class I are typically 8 to 11 amino acids in length, whereas CD4+ T cells are activated by longer epitopes (12-25 amino acids) presented in the context of major histocompatibility complex class II.38 In this study, we found that HBZ157-176 is an immunodominant peptide, on the basis of results from rVV-vaccinated mice and HTLV-1–infected monkeys. Furthermore, this peptide induces the activation of both HBZ-specific CD4+ T cells and CD8+ T cells in vaccinated mice (supplemental Figure 5B), suggesting that HBZ157-176 has the potential to be used in peptide-based vaccines for HTLV-1 infection.
As shown in this study, the immunogenicity of HBZ protein is low compared with Tax protein. One reason for the weak CTL response to HBZ protein might be that its expression is low in vivo.39 It has been reported that HBZ RNA tends to be retained in the nucleus,40 which might reduce HBZ protein production. However, as shown in this study, repeated immunization that causes overproduction of HBZ protein in vivo does lead to a CTL response. It is noteworthy that ELISPOT assay could not detect CTLs in HTLV-1–infected rhesus macaques. However, their immunization with rVV-expressing HBZ enables detection of CTLs to HBZ. These findings indicate that immunizing with a vector that produces the right amount of HBZ protein can overcome the weak production of HBZ protein by HTLV-1–infected cells and ATL cells and trigger an immune response. In addition, because regulatory T cells control CTL response in HTLV-1–infected individuals,41 inhibition of their function in vivo by monoclonal antibody might enhance immune responses to HBZ.42 Alternatively, amino acid substitution of epitopes could enhance CTL development by increasing binding affinity to HLA molecules.43 Considering that splenocytes transferred from HBZ-immunized mice partially rescued Ht48-inoculated mice, vaccinating with HBZ appears to be a viable therapeutic choice for ATL patients.
In this study, we generated a new HBZ-induced T-cell cell line, Ht48, from a T-cell lymphoma in an HBZ-Tg mouse. After several in vivo passages, this cell line could be cultured in vitro, and it induced lethal lymphomas in inoculated WT C57BL/6 mice (Figures 3 and 4). Like the Ht48 cell line, some ATL cell lines express only HBZ; Tax expression is inactivated in those cells by deletion of the 5′ long terminal repeat, DNA methylation of the 5′ long terminal repeat, or genetic changes of the tax gene.21 Similarly, it has been reported that Tax expression is inactivated in some ATL patients, whereas the HBZ gene is expressed in all ATL patients.15,21 Therefore, Ht48 likely corresponds to ATL cells that express only the HBZ gene. Another T-cell line obtained from the leukemia cells of a Tax-transgenic mouse was described in a previous study.44 Such mouse T-cell lines that express only Tax or HBZ may be useful tools for studying the pathogenesis of ATL and the oncogenic roles of these viral genes.
In this study, we generated HBZ-expressing rVV to effectively induce T-cell memory responses in vivo as a potential new therapeutic tool. CTL responses to HBZ could prolong the survival of Ht48-inoculated mice, suggesting that a vaccine targeting HBZ might be useful as a therapeutic tool to control ATL or prevent its development in HTLV-1 infected individuals.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Dantsuka, H. Sakawaki, N. Izawa, K. Mizuta, and J. Tanabe for technical support of the monkey experiments; Y. Hirata, S. Naganawa, and F. Yasui for technical support in generating recombinant virus; T. Kitamura for the pMXs-Ig and Plat-E cells; A. Shimizu for the Ly5.1 mice; S. Shoji for the pIRES-hrGFP-1a; L. Kingsbury for proofreading the manuscript; and H. Nishikawa, D. Sugiyama, K. Shimura, T. Zhao, A. Rowan, Y. Satou, Y. Nishimua, and C.R. Bangham for valuable advice on experiments.
This work was supported by grants 22114003 (M. Matsuoka) and 26860301 (K.S.) from the Ministry of Education, Culture, Sports, Science and Technology Japan (MEXT), and a grant from the Japan Leukemia Research Fund (M. Matsuoka). This study was performed as part of a research program of the Project for Development of Innovative Research on Cancer Therapeutics, MEXT (J.-i.Y.) and was also supported in part by the Japan Society for the Promotion of Science Core-to-Core Program A, Advanced Research Networks, and by the Joint Usage/Research Center program of Institute for Virus Research, Kyoto University.
Authorship
Contribution: K.S., J.-i.Y., M. Miura, M.K., and M. Matsuoka conceived and designed the experiments; K.S., Y.M., and P.M. performed the experiments; K.S., J.-i.Y., M.K., and M. Matsuoka analyzed the data; Y.M., P.M., and M.K. contributed reagents, materials, and analysis tools; and K.S., J.-i.Y., and M. Matsuoka wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masao Matsuoka, Laboratory of Virus Control, Institute for Virus Research, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: mmatsuok@virus.kyoto-u.ac.jp.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal