In this issue of Blood, Gros et al1 report that glycoprotein VI (GPVI) promotes the proinflammatory role of platelets by increasing neutrophil secretion and toxicity while at the same time repairing the vascular damage inflicted by neutrophil activation, thereby maintaining vascular integrity. Significantly, this effect is independent of hemostasis.
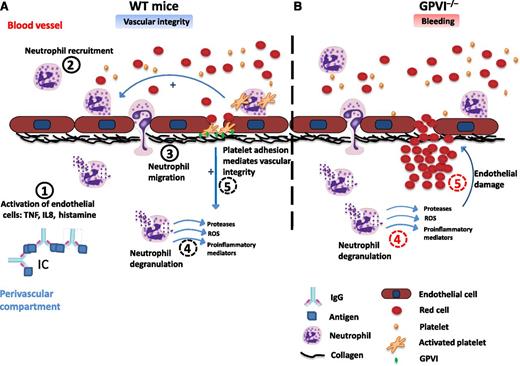
Proposed mechanism for platelet GPVI in maintaining vascular integrity during immune-complex–mediated inflammation. (A) Proposed mechanism for the role of platelets in wild-type (WT) mice. (A1) Immune complex formation in the perivascular compartment induces endothelial cells activation mainly through the release of proinflammatory cytokines and histamine from activated immune cells. (A2) Neutrophils are recruited to the activated endothelium, adhere, and transmigrate to the inflamed tissue. (A3,4) Platelets, including activation of GPVI, enhance neutrophil recruitment, infiltration, degranulation, and oxidative stress. (A5) Single platelets adhere to the subendothelium and seal the neutrophil-mediated vascular damage. (B) Proposed mechanism for the role of platelets in GPVI−/− mice. (B4) Neutrophil recruitment, infiltration, and degranulation in the inflamed tissue induce vascular damage, although this is partly reduced by the absence of GPVI. (B5) Platelets are not able to adhere and seal neutrophil-mediated vascular damage in the absence of GPVI, thereby increasing blood leakage and local hemorrhage. IC, immune complex; ROS, reactive oxygen species; TNF, tumor necrosis factor; IL8, interleukin-8; IgG, immunoglobulin G.
Proposed mechanism for platelet GPVI in maintaining vascular integrity during immune-complex–mediated inflammation. (A) Proposed mechanism for the role of platelets in wild-type (WT) mice. (A1) Immune complex formation in the perivascular compartment induces endothelial cells activation mainly through the release of proinflammatory cytokines and histamine from activated immune cells. (A2) Neutrophils are recruited to the activated endothelium, adhere, and transmigrate to the inflamed tissue. (A3,4) Platelets, including activation of GPVI, enhance neutrophil recruitment, infiltration, degranulation, and oxidative stress. (A5) Single platelets adhere to the subendothelium and seal the neutrophil-mediated vascular damage. (B) Proposed mechanism for the role of platelets in GPVI−/− mice. (B4) Neutrophil recruitment, infiltration, and degranulation in the inflamed tissue induce vascular damage, although this is partly reduced by the absence of GPVI. (B5) Platelets are not able to adhere and seal neutrophil-mediated vascular damage in the absence of GPVI, thereby increasing blood leakage and local hemorrhage. IC, immune complex; ROS, reactive oxygen species; TNF, tumor necrosis factor; IL8, interleukin-8; IgG, immunoglobulin G.
In 2008, the laboratory of Denisa Wagner demonstrated an unexpected role for platelets in prevention of bleeding at sites of inflammation using dermatitis, lung, and stroke models. In their seminal paper, Goerge et al2 demonstrated that bleeding is only seen on inflammatory challenge when the platelet count is reduced by over 95%.2 Not only was the presence of hemorrhaging surprising, but it was also not seen in mice lacking the major glycoprotein receptors, GPIIbIIIa, GPIbα, and GPVI–Fc receptor (FcR) γ-chain complex, or the regulator of platelet activation, CALDAG-GEFI, indicating that it was independent of hemostasis.
A breakthrough in the understanding of this unexpected role of platelets subsequently came from the laboratory of Wolfgang Bergmeier.3 Using a novel adoptive transfer approach to introduce genetically or chemically modified platelets into thrombocytopenic mice, Boulaftali et al3 reported that hemorrhaging in lung and skin models was not rescued by platelets deficient in GPVI or CLEC-2, whereas recovery was seen on introduction of platelets with a combined deficiency of the major G-protein–coupled receptors for thrombin, adenosine 5′-diphosphate, and thromboxane A2.3 This study establishes a unique role for the 2 immunoreceptor tyrosine-based activation motif (ITAM) receptors, which signal through Src, Syk, and Tec family kinases, in vascular integrity (ie, maintenance of barrier function). The contrasting conclusion on the role of GPVI reached by Goerge et al2 is explained by the use of the FcR γ-chain–deficient mouse model, which is deficient in both GPVI and the ITAM-containing subunit. Thus, it would appear that the FcR γ-chain is essential for the inflammation to occur.
The study of Boulaftali et al3 strongly suggests that the protective effect is triggered by binding of platelets to subendothelial collagen fibers and possibly the CLEC-2 ligand podoplanin. Podoplanin is normally absent in the vessel wall but is upregulated during inflammation in several cell types, including macrophages4 and Th-17 cells.5 Indeed, it may be that upregulation of podoplanin deep into the vessel wall prevents damage to GPVI at distinct sites. Alternatively, there may a second, as yet undiscovered ligand for CLEC-2 in the vessel wall.
This study, however, did not provide a molecular basis for the protective roles of GPVI and CLEC-2, and it is this question that is addressed by Gros et al1 in the current issue. Gros et al1 focused on the molecular mechanism underlying the role of GPVI in the skin reverse passive Arthus (rpA) reaction as used by Goerge et al2 and Boulaftali et al.3 They firstly confirmed the increase in bleeding in the rpA model in thrombocytopenic mice and further demonstrated that bleeding is also seen in GPVI-deficient mice, which have a normal platelet count. Moreover, they showed that bleeding was prevented by depletion of neutrophils by over 70%.
As neutrophil-mediating tissue injury is a multistep process, Gros et al1 investigated the effect of platelets on neutrophil mobilization, recruitment, activation, secretion, and oxidative stress. Although platelets did not induce neutrophil mobilization, they increased neutrophil infiltration at the site of inflammation independent of GPVI. Many potential pathways could underlie this role of platelets, including the interaction of platelet P-selectin with PSGL1 and through chemokine release.6 Platelet activation by GPVI and other ligands, however, was shown to induce neutrophil granule secretion and oxidative activity, contributing to tissue injury. On the other hand, GPVI was also shown to have a counter role by sealing the sites of damage in the inflamed vessel wall and thus preventing neutrophil-induced bleeding (see figure). It was unclear, however, if this is due to formation of an occlusive barrier, a novel mode of hemostasis (that is independent of GPIIbIIIa), or altered endothelial function.
Together, these studies establish a critical role for platelet GPVI in the maintenance of vascular integrity independent of classical hemostasis. This may therefore explain the spontaneous bleeding observed in thrombocytopenic patients.
It is tempting to speculate that this may be the major physiological role of GPVI, as patients and mouse models deficient in the immunoglobulin receptor present with mild or no apparent defects in hemostasis. On the other hand, GPVI-deficient patients or genetically targeted mice do not have widespread hemorrhaging, suggesting the presence of an alternative mechanism. On the basis of the work of Boulaftali et al,3 this is likely to be mediated by CLEC-2 and possible upregulation of podoplanin.
The critical roles of the 2 ITAM receptors in vascular integrity may have pathological counterparts in a variety of vascular inflammatory conditions ranging from the onset of atherosclerosis to ischemic stroke, and this, together with their relatively minor roles in hemostasis, renders GPVI and CLEC-2 as highly attractive novel therapeutic targets.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal