Key Points
2-CdA is an effective treatment with a long-term acceptable safety profile in patients with mastocytosis.
2-CdA is effective and safe in indolent systemic mastocytosis and cutaneous mastocytosis refractory to multiple symptomatic therapies.
Abstract
Mastocytosis (M) is a clonal myeloid-disabling disorder for which no curative therapy is currently available. Cladribine (2-chlorodeoxyadenosine [2-CdA]) is a synthetic purine analog cytoreductive treatment, for which efficacy is mostly reported in advanced M. Here we report, with a long-term follow-up period (>10 years) efficacy and safety in 68 adult patients with M (36 [53%] had indolent M and 32 [47%] had advanced M) treated by 2-CdA (0.14 mg/kg in infusion or subcutaneously, days 1-5; repeated at 4-12 weeks until 1 to 9 courses). Median 2-CdA courses number was 3.7 (1-9). The overall response rate was 72% (complete remission [R]/major/partial R: 0%/47%/25%) and according to indolent/advanced M was 92% (major/partial R: 56%/36%) and 50% (major/partial R: 37.5%/12.5%), respectively. Clinical improvement was observed for 10 of 11 mediator release and 6 of 7 mast cell infiltration–related symptoms including urticaria pigmentosa and organomegaly (P < .02). Serum tryptase levels decreased (P = .01). Median durations of response were 3.71 (0.1-8) and 2.47 (0.5-8.6) years for indolent and aggressive M, respectively. The most frequent grade 3/4 toxicities were lymphopenia (82%), neutropenia (47%), and opportunistic infections (13%). 2-CdA appears to provide a significant efficacy with some toxicity in various M subtypes, mostly in indolent M, refractory to multiple symptomatic therapies.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1050.
Disclosures
Associate Editor Jacob M. Rowe served as an advisor or consultant for Amgen. The authors and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the efficacy of cladribine (2-chlorodeoxyadenosine [2-CdA]) in indolent mastocytosis.
Describe the efficacy of 2-CdA in advanced mastocytosis.
Distinguish the toxicity of 2-CdA in various subtypes of mastocytosis.
Release date: August 20, 2015; Expiration date: August 20, 2016
Introduction
Mastocytosis (M) is a clonal myeloid-disabling disorder for which no curative therapy is currently available.1 Although not specifically registered for M, several cytoreductive therapies are used in adults with systemic mastocytosis (SM) comprising interferon-α,2 hydroxyurea,3 tyrosine kinase inhibitors (TKI),4-6 thalidomide,7 midostaurin (PKC412),8-10 and cladribine (2-chlorodeoxyadenosine [2-CdA]).3 2-CdA is a synthetic purine analog that inhibits DNA repair, actively blocks dividing cells, and induces apoptosis in resting cells.11 2-CdA is toxic for monocytes in vitro and in vivo. Although not confirmed with strong experimental data, at the time of the beginning of this study, it was suggested that mast cells and monocytes derive from a common precursor stem cell. Thus, we have considered using 2-CdA for mastocytosis treatment as it was done in Langerhans cell histiocytosis.12,13 Likewise, since the beginning of our study, several publications have demonstrated the efficacy of 2-CdA in reducing the mast cell burden in limited series of patients with a short follow-up, mostly in advanced mastocytosis comprising aggressive systemic mastocytosis (ASM) and SM with an associated clonal hematologic non–mast cell lineage disease (SM-AHNMD).3,14-16 Tefferi et al described the first case of a patient with ASM treated by 2-CdA in 2001 with major response.16 Kluin-Nelemans et al in 2003 reported a series of 10 patients with 4 indolent M (3 ISM and 1 smoldering systemic mastocytosis [SSM] patients) and 6 advanced M (3 ASM, and 3 SM-AHNMD) also with significant improvement and good safety profile.14 Pardanani et al in 2004 has then reported 4 ASM patients with an ORR of 75%15 and Bohm et al in 2010 confirmed an ORR of 50% in 4 ASM and of 66% in 3 ISM patients.11 The largest series published by Lim et al in 2009 has analyzed 30 patients treated by 2-CdA with an ORR ranging from 50% to 56% according to M subtypes.3 However, few patients with indolent systemic mastocytosis (ISM), smoldering systemic mastocytosis (SSM), or cutaneous mastocytosis (CM) with disabling mediator-related symptoms refractory to symptomatic treatments have been treated with 2-CdA and reported in the current literature. In addition, long-term follow-up data regarding efficacy and particularly safety of 2-CdA treatment are lacking in all M subtypes. In the present study, the national reference center for mastocytosis in France (CEREMAST) evaluated the efficacy and safety of 2-CdA in 68 adult patients with CM, ISM, SSM, ASM, and SM-AHNMD over the last decade.
Patients and methods
Study design and patient eligibility
Between March 2001 and June 2010, 68 adult patients with a mastocytosis diagnosis according to 2001 consensus World Health Organization (WHO) criteria17,18 registered at the CEREMAST originating from various university centers, and treated by at least 1 course of 2-CdA, were included in a nationwide retrospective study. The ethical committee of Necker-Enfants Malades Hospital (CPP “Ile de France II” reference number DA 2011-078) approved this study. Patients were classified as having ISM (n = 28, 41%), SSM (n = 2, 3%), ASM (n = 14, 21%), SM-AHNMD (n = 17, 25%), MCL (n = 1, 1%), and CM (n = 6, 9% including 3 patients not investigated for bone marrow biopsy and subsequently defined as mastocytosis in the skin [MIS] patients). Among SM-AHNMD, 11 (16%) were ASM-AHNMD and 6 (9%) were ISM-SSM/AHNMD. AHNMD variants displayed myelodysplastic syndrome (MDS) (n = 6)/myeloproliferative neoplasm (n = 4), chronic myelomonocytic leukemia (n = 4), hairy cell leukemia (HCL) (n = 1) and non-Hodgkin lymphoma (n = 3). According to the respective prognosis of the mastocytosis subtypes, CM, ISM, and SSM were grouped in the “indolent mastocytosis” group, whereas ASM and AHNMD (whatever associated hematologic malignancy) were grouped in the “advanced mastocytosis” group). History of mastocytosis, clinical symptoms, and biological data were recorded at baseline, after each course, and 2 months after the last treatment’s course. Clinical characteristics included mediators release symptoms19 and mast cells infiltration symptoms. C-findings were taken into account as previously described.17 Biological data included serum tryptase level, complete blood cell count, liver parameters (total bilirubin, serum transaminases, and alkaline phosphatase levels), and molecular analysis for KIT D816V mutation in bone marrow aspirate or in skin lesions as previously described.20 All patients were refractory to multiple symptomatic treatments (histamine H1 and H2 blockers ± sodium cromoglycate and proton-pump inhibitor) for indolent mastocytosis and 50% (indolent [23 pts] or advanced [11pts]) mastocytosis patients had previous mastocytosis cytoreductive therapies. Clinical history of patients was collected until October 30, 2011.
Treatment
Treatment consisted of 2-CdA (0.14 mg/kg/d) administered over a 2-hour infusion (Leustatine) or subcutaneously (Litak) from 1 to 5 days, which defined a course of treatment. Each course was repeated depending on the treating practitioner’s judgment within a 4- to 12-week intervals from 1 to a maximal number of 9 consecutive courses. All patients had systematic antiinfective prophylaxis with both cotrimoxazole and valaciclovir during the treatment period and 18 months beyond the last course.
Assessments
Response was the principal efficacy criterion and was systematically evaluated within 2 months after the last 2-CdA course in accordance to the consensus statement for response criteria for mastocytosis.3,21 This evaluation differed according to mastocytosis subtypes as described in the series of Lim et al.3 Briefly, complete response (CR) included complete resolution of all symptoms (cutaneous and general symptoms) and signs lasting at least 1 month; major response (MR) included a >50% improvement in symptoms and signs; partial response (PR) comprised at least 10% to 50% improvement. The overall response (OR) was defined as the sum of CR, MR, and PR. Progression was defined as worsening from CR to MR, MR to PR, or PR to NR. If one of the symptoms worsened, patients were classified as in progression even though some other symptoms improved. Bone marrow biopsy and skin biopsy of urticaria pigmentosa lesions were also analyzed when available by KIT staining (clone CD117; DAKO, Copenhagen, Denmark) with evaluation of variations either of percent in bone mineral density or mast cells count per mm2 in the dermis, respectively. Among those who had a relapse, some patients were evaluated again after 2-CdA retreatment. Safety was recorded for all patients, taking into account that 2 patients were lost to follow-up at 1 and 6 months after the last 2-CdA course. Acute toxicity occurring during 2-CdA courses was recorded and differentiated from late-occurring toxicity up to 3 months after the last dose of 2-CdA. The severity of neutropenia and lymphopenia was scored following the grading system analysis (CTCAE V3).22 We focused also on subsequent malignancies observed during follow-up. Mortality and cause of death were recorded and related to mastocytosis, 2-CdA therapy, or to any other origin.
Statistical procedures
Paired comparisons of clinical and biological parameters available between baseline and end of 2-CdA therapy were performed using the McNemar’s χ2 test or the Wilcoxon signed-rank test. Comparisons between mastocytosis subtypes parameters were performed using the Fisher exact test or χ2 test for qualitative variables, and using the Wilcoxon rank-sum test for continuous variables.
Because of heterogeneity of treatment and the practitioner’s decision about whether to pursue the treatment (ie, an additional course), progression-free survival data were not provided. However, we were able to define a relapse-free survival (RFS) and duration of response as the time between the date of the last consecutive course of 2-CdA considered for end point response and relapse or death or last follow-up, as appropriate.
Overall survival (OS) was defined as the time between the date of 2-CdA initiation and death (as a result of all causes) or last follow-up.
Both RFS and OS were estimated by the Kaplan-Meier method and survivals comparisons were performed by the log-rank test. Hazard ratios (HR) associated with PFS or OS were calculated with their 95% confidence intervals (CI) for each covariate with the Cox proportional hazards regression model. The full multivariate model includes covariates significantly associated with survival with a significance of P < .05 in univariate analysis. Then, backward elimination (P < .05) was used to select covariates to retain in the final multivariable analysis.
All statistical analyses were performed using R software (http://cran.r-project.org). Statistical significance was considered as P < .05 and all tests were two-sided.
Results
Patient characteristics
The demographic, clinical, and biological data of the 68 study patients are summarized in Table 1. Patients were 51% female with a mean age at 2-CdA treatment initiation of 54 ± 20.5 years. The median time from mastocytosis diagnosis to 2-CdA initiation was 47 months (range, 1-473). Patients had indolent mastocytosis in 36 cases (53%) and advanced mastocytosis in 32 cases (47%). Mediators release frequent symptoms were: fatigue (79%), flush (53%), pruritus (53%), diarrhea (53%), and abdominal pain (48.5%). Mast cells infiltration-related frequent symptoms consisted of: urticaria pigmentosa (75%), bone involvement (62%), hepatomegaly (51%), and splenomegaly (48.5%). Serum tryptase level was >20 ng/mL in 87% of tested patients, with a median value of 95 ng/mL (range, 20.2-1240), similar in indolent and advanced mastocytosis (P = .41).
Baseline demographic, clinical, and biological characteristics of 68 patients with mastocytosis treated by 2-CdA
| Characteristics . | No. of patients (%) . | Median (range) . |
|---|---|---|
| Total no. of patients | 68 | |
| WHO subtypes | ||
| Indolent mastocytosis | 36 (53) | |
| CM/MIS | 6 (9) | |
| ISM | 28 (41) | |
| SSM | 2 (3) | |
| Advanced mastocytosis | 32 (47) | |
| ASM | 14 (21) | |
| SM-AHNMD | 17 (25) | |
| MCL | 1 (1.4) | |
| Female | 35 (51) | |
| Age at treatment’s initiation, y | 54 (17-83) | |
| Time from diagnosis to treatment, mo | 47 (1-473) | |
| Time from appearance of symptoms to 2-CdA’s initiation, mo | 160 (2-473) | |
| Clinical findings | ||
| Mediators release symptoms | ||
| Fatigue | 54 (79) | |
| Flush | 36 (53) | |
| Pruritus | 36 (53) | |
| Diarrhea | 36 (53) | |
| Abdominal pain | 33 (48.5) | |
| Neuropsychiatric symptoms | 28 (41) | |
| Headache/chronic pain | 24 (35) | |
| Nausea, vomiting | 21 (30) | |
| Dyspnea | 14 (20.5) | |
| Mast cells infiltration-related symptoms | ||
| Urticaria pigmentosa | 51 (75) | |
| Bone involvement (osteopenia, osteoporosis, compression fracture, osteosclerosis) | 42 (62) | |
| Hepatomegaly | 35 (51) | |
| Splenomegaly | 33 (48.5) | |
| Weight loss, fever, chills, night sweats (B-symptoms) | 25 (36.7) | |
| Lymphadenopathy | 18 (26) | |
| Ascites | 10 (15) | |
| Biological findings | ||
| Hemoglobin, g/dL | 64 (94) | 12.1 (6.5-15.8) |
| Hb <10 | 18 (28) | 8.9 (6.5-9.7) |
| White blood cell count, ×109/L | 64 (94) | 6.7 (2.1-108) |
| WBC >10 | 10 (16) | 13.1 (10.6-108) |
| Absolute neutrophil count | 63 (93) | 3.8 (0.49-14) |
| ANC <1 | 2 (3) | 0.7 (0.4-0.9) |
| Absolute eosinophil count, ×109/L | 58 (85) | 0.2 (0-99.9) |
| AEC >0.5 | 14 (24) | 1.88 (0.6-99.9) |
| Platelet count, ×109/L | 65 (96) | 215 (16-966) |
| Platelet <100 | 14 (22) | 52 (16-87) |
| Liver parameters (total bilirubin, SAP, AST/ALT > UNL) | 25 (37) | — |
| Albumin, >35-55 g/L | 39 (57) | 43 (35-53) |
| Albumin <35 | 8 (20) | 30 (22.7-32.9) |
| Serum tryptase, ng/mL | 54 (80) | 78 (2.7-1240) |
| Serum tryptase >20 | 47 (87) | 95 (20.2-1240) |
| C-Findings | 26 (38) | |
| KIT D816V mutation detected | 58 (81) | — |
| From bone marrow aspiration in 25 patients | 19 (76) | — |
| From skin biopsy in 49 patients | 39 (80) | — |
| Previous mastocytosis therapies | 34 (50) | |
| Interferon-α | 13 (19) | |
| TKIs (imatinib, masitinib, dasatinib, imatinib then masitinib) | 6/6/1/3 | |
| Thalidomide | 5 (7) | |
| Hydroxyurea | 3 (4) |
| Characteristics . | No. of patients (%) . | Median (range) . |
|---|---|---|
| Total no. of patients | 68 | |
| WHO subtypes | ||
| Indolent mastocytosis | 36 (53) | |
| CM/MIS | 6 (9) | |
| ISM | 28 (41) | |
| SSM | 2 (3) | |
| Advanced mastocytosis | 32 (47) | |
| ASM | 14 (21) | |
| SM-AHNMD | 17 (25) | |
| MCL | 1 (1.4) | |
| Female | 35 (51) | |
| Age at treatment’s initiation, y | 54 (17-83) | |
| Time from diagnosis to treatment, mo | 47 (1-473) | |
| Time from appearance of symptoms to 2-CdA’s initiation, mo | 160 (2-473) | |
| Clinical findings | ||
| Mediators release symptoms | ||
| Fatigue | 54 (79) | |
| Flush | 36 (53) | |
| Pruritus | 36 (53) | |
| Diarrhea | 36 (53) | |
| Abdominal pain | 33 (48.5) | |
| Neuropsychiatric symptoms | 28 (41) | |
| Headache/chronic pain | 24 (35) | |
| Nausea, vomiting | 21 (30) | |
| Dyspnea | 14 (20.5) | |
| Mast cells infiltration-related symptoms | ||
| Urticaria pigmentosa | 51 (75) | |
| Bone involvement (osteopenia, osteoporosis, compression fracture, osteosclerosis) | 42 (62) | |
| Hepatomegaly | 35 (51) | |
| Splenomegaly | 33 (48.5) | |
| Weight loss, fever, chills, night sweats (B-symptoms) | 25 (36.7) | |
| Lymphadenopathy | 18 (26) | |
| Ascites | 10 (15) | |
| Biological findings | ||
| Hemoglobin, g/dL | 64 (94) | 12.1 (6.5-15.8) |
| Hb <10 | 18 (28) | 8.9 (6.5-9.7) |
| White blood cell count, ×109/L | 64 (94) | 6.7 (2.1-108) |
| WBC >10 | 10 (16) | 13.1 (10.6-108) |
| Absolute neutrophil count | 63 (93) | 3.8 (0.49-14) |
| ANC <1 | 2 (3) | 0.7 (0.4-0.9) |
| Absolute eosinophil count, ×109/L | 58 (85) | 0.2 (0-99.9) |
| AEC >0.5 | 14 (24) | 1.88 (0.6-99.9) |
| Platelet count, ×109/L | 65 (96) | 215 (16-966) |
| Platelet <100 | 14 (22) | 52 (16-87) |
| Liver parameters (total bilirubin, SAP, AST/ALT > UNL) | 25 (37) | — |
| Albumin, >35-55 g/L | 39 (57) | 43 (35-53) |
| Albumin <35 | 8 (20) | 30 (22.7-32.9) |
| Serum tryptase, ng/mL | 54 (80) | 78 (2.7-1240) |
| Serum tryptase >20 | 47 (87) | 95 (20.2-1240) |
| C-Findings | 26 (38) | |
| KIT D816V mutation detected | 58 (81) | — |
| From bone marrow aspiration in 25 patients | 19 (76) | — |
| From skin biopsy in 49 patients | 39 (80) | — |
| Previous mastocytosis therapies | 34 (50) | |
| Interferon-α | 13 (19) | |
| TKIs (imatinib, masitinib, dasatinib, imatinib then masitinib) | 6/6/1/3 | |
| Thalidomide | 5 (7) | |
| Hydroxyurea | 3 (4) |
ALT, alanine aminotransferase; ASM, aggressive systemic mastocytosis; AST, aspartate aminotransferase; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; MCL, mast cell leukemia; MIS, mastocytosis in the skin; SAP, serum alkaline phosphatase; SM-AHNMD, systemic mastocytosis with an associated clonal hematologic non–mast cell lineage disease; SSM, smoldering systemic mastocytosis; TKI, tyrosine kinase inhibitors; UNL, upper normal limit.
Therapeutic modalities
Patients received 2-CdA IV (Leustatine; Janssen-Cilag, Issy-les-Moulineaux, France) in 51% of cases or subcutaneously (Litak; Lipomed GmbH, Weil/Rhein, Germany) in 49% of cases. Patients with advanced mastocytosis more frequently received IV administration than did those with indolent mastocytosis (P = .013). The median 2-CdA number of courses received was 3.68 (range, 1-9). The cumulative median dose of 2-CdA received by patients at the time of evaluation was 2.25 mg/kg (range, 0.45- 4.95). The median time interval between consecutive courses for patients receiving at least 2 courses was 52 (range, 28-83) days. Two patients were lost to follow-up at 1 and 16 months after 2-CdA, after 1 and 2 courses, respectively.
Efficacy
Response to treatment.
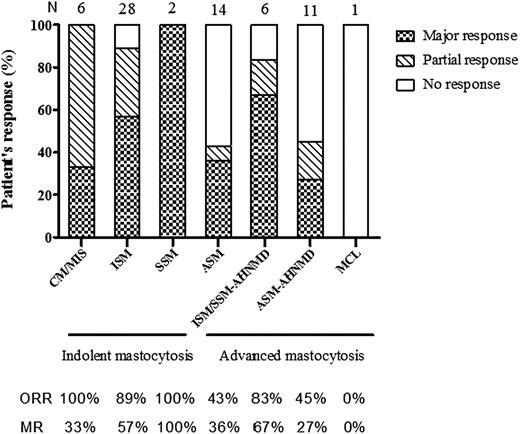
An OR rate of 72% (49/68 pts) was observed comprising 47% (32/68 pts) of MR and 25% (17/68 pts) of PR but without any CR. The OR rates according to M subtypes were 92% (CR/MR/PR: 0%/56%/36%) and 50% (CR/MR/PR: 0%/37.5%/12.5%) in indolent and advanced M, respectively. Percentages of OR, MR, PR, and NR according to mastocytosis subtypes are shown in Figure 1. The highest response rate was observed significantly in indolent mastocytosis (ISM [89%], SSM [100%], and CM [100%]) (92%) compared with ASM (43%) and SM-AHNMD (59%) (P < .001). Clinical responses for mediator release and mast cell infiltration-related symptoms are listed in Table 2. There was no statistical difference between the route of administration and the type of clinical response (P = .11). Most clinical mediator release symptoms (10/11) significantly improved (P < .02). Mast cell infiltration-related symptoms also significantly improved (5/6) including urticaria pigmentosa, hepatomegaly, splenomegaly (B-signs), weight loss/fever/chills/night sweats, and ascites (P < .02). We observed an urticaria pigmentosa OR for 38 patients (80%) comprising 11 CR, 17 MR, and 10 PR (supplemental Figure 1, available on the Blood Web site).
2-CdA responses (symptoms + infiltration) according to mastocytosis subtypes. ASM, aggressive systemic mastocytosis; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; MCL, mast cell leukemia; MIS, mastocytosis in the skin; MR, major response; N, number of patients evaluated for 2-CdA response in each WHO mastocytosis subtype; ORR, overall response rate; SM (ISM, SSM, ASM)-AHNMD, systemic mastocytosis with an associated clonal hematological non–mast cell lineage disease; SSM, smoldering systemic mastocytosis.
2-CdA responses (symptoms + infiltration) according to mastocytosis subtypes. ASM, aggressive systemic mastocytosis; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; MCL, mast cell leukemia; MIS, mastocytosis in the skin; MR, major response; N, number of patients evaluated for 2-CdA response in each WHO mastocytosis subtype; ORR, overall response rate; SM (ISM, SSM, ASM)-AHNMD, systemic mastocytosis with an associated clonal hematological non–mast cell lineage disease; SSM, smoldering systemic mastocytosis.
Clinical and biological data before and at the end of 2-CdA treatment
| Clinical data responses . | No. of patients before treatment (%) . | No. of patients after treatment (%) . | P . |
|---|---|---|---|
| Mediators release symptoms | |||
| Fatigue | 53 (79) | 22 (33) | <.0001 |
| Flush | 34 (52) | 11 (17) | <.0001 |
| Pruritus | 33 (51) | 16 (25) | <.0001 |
| Diarrhea | 37 (55) | 9 (13) | <.0001 |
| Abdominal pain | 33 (49) | 12 (18) | <.0001 |
| Neuropsychiatric symptoms | 24 (37) | 13 (20) | .0009 |
| Headache/pain | 23 (34) | 15 (22) | .0046 |
| Nausea, vomiting | 22 (33) | 8 (12) | .0001 |
| Dyspnea | 14 (21) | 5 (7) | .007 |
| Anaphylaxis | 7 (10) | 0 (0) | .02 |
| Pollakiuria | 9 (14) | 5 (8) | .13 |
| Mast cell infiltration-related symptoms | |||
| Urticaria pigmentosa | 48 (73) | 10 (15) | <.0001 |
| Hepatomegaly | 34 (51) | 24 (36) (+1)* | .006 |
| Splenomegaly | 32 (48) | 24 (36) | .013 |
| Weight loss, fever, chills, night sweats (B-signs) | 25 (37) | 9 (13) | <.0001 |
| Lymphadenopathy | 18 (27) | 15 (22) (+2)* | .44 |
| Ascites | 15 (22) | 8 (12) | .023 |
| Biological parameters | |||
| Hemoglobin, g/dL | |||
| Hb <10 | 18 (27) | 13 (19) | .07 |
| Absolute eosinophil count, ×109/L | |||
| AEC >.5 | 14 (24) | 12 (21) | .5 |
| Platelet count, ×109/L | |||
| Platelet <100 | 14 (22) | 13 (19) | .24 |
| Liver parameters (total bilirubin, SAP, AST, ALT > UNL) | 9 (13) | 7 (10) (+1)* | .47 |
| Serum tryptase, ng/mL | |||
| Mean value | 172 | 97 | .01 |
| Median value | 79 | 53 |
| Clinical data responses . | No. of patients before treatment (%) . | No. of patients after treatment (%) . | P . |
|---|---|---|---|
| Mediators release symptoms | |||
| Fatigue | 53 (79) | 22 (33) | <.0001 |
| Flush | 34 (52) | 11 (17) | <.0001 |
| Pruritus | 33 (51) | 16 (25) | <.0001 |
| Diarrhea | 37 (55) | 9 (13) | <.0001 |
| Abdominal pain | 33 (49) | 12 (18) | <.0001 |
| Neuropsychiatric symptoms | 24 (37) | 13 (20) | .0009 |
| Headache/pain | 23 (34) | 15 (22) | .0046 |
| Nausea, vomiting | 22 (33) | 8 (12) | .0001 |
| Dyspnea | 14 (21) | 5 (7) | .007 |
| Anaphylaxis | 7 (10) | 0 (0) | .02 |
| Pollakiuria | 9 (14) | 5 (8) | .13 |
| Mast cell infiltration-related symptoms | |||
| Urticaria pigmentosa | 48 (73) | 10 (15) | <.0001 |
| Hepatomegaly | 34 (51) | 24 (36) (+1)* | .006 |
| Splenomegaly | 32 (48) | 24 (36) | .013 |
| Weight loss, fever, chills, night sweats (B-signs) | 25 (37) | 9 (13) | <.0001 |
| Lymphadenopathy | 18 (27) | 15 (22) (+2)* | .44 |
| Ascites | 15 (22) | 8 (12) | .023 |
| Biological parameters | |||
| Hemoglobin, g/dL | |||
| Hb <10 | 18 (27) | 13 (19) | .07 |
| Absolute eosinophil count, ×109/L | |||
| AEC >.5 | 14 (24) | 12 (21) | .5 |
| Platelet count, ×109/L | |||
| Platelet <100 | 14 (22) | 13 (19) | .24 |
| Liver parameters (total bilirubin, SAP, AST, ALT > UNL) | 9 (13) | 7 (10) (+1)* | .47 |
| Serum tryptase, ng/mL | |||
| Mean value | 172 | 97 | .01 |
| Median value | 79 | 53 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAP, serum alkaline phosphatase; UNL, upper normal limit.
(+n) corresponds to the appearance of clinical or biological sign in patient(s) without the biological sign at baseline.
Biological responses are listed in Table 2. In contrast to clinical symptoms, biological parameters did not improve significantly, except tryptase serum levels, which decreased significantly (P= .01) among 27 paired patients before and after treatment. Of note, the decrease of tryptase level was observed only in indolent mastocytosis (P = .0004).
Bone marrow responses in MR patients are illustrated in supplemental Figure 2. Overall, only 9 pairs of bone marrow biopsy for MR patients were available. The analysis showed a significantly decreased percentage of KIT-stained mast cells (from a median of 32% initially to 11% subsequently, P = .008) after 2-CdA without any complete pathologic response. These patients were 3 ISM, 1 SSM, 2 ASM, and 3 ISM-AHNMD cases.
Among 17 MR patients for urticaria pigmentosa, only 3 pairs of skin biopsy before and after 2-CdA were available for analysis, with a median number of decrease from 136 to 85 mast cells per mm2. Skin biopsy pathology evaluations in MR patients are illustrated in supplemental Figure 3.
The number of courses according to mastocytosis subtypes was not significantly different. The median number of 2-CdA courses significantly differed for patients with OR (median 4 [range, 1-9]) compared with those who did not respond (median 3 [range, 1-6]) (P= .048).
Outcome.
Relapse-free survival.
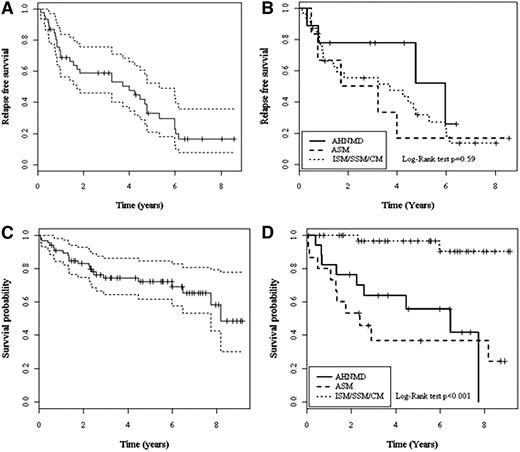
Among the 49 patients with OR, 48 had follow-up information. Median time of follow-up after 2-CdA was 5.8 years (range, 21 d-9 y, 95% CI, 4.7-6.9). Among them, 29 had a relapse (60%) and 4 died (8%). The RFS was 23.4% (95% CI, 10.7-51.2) (Figure 2A). The median duration of RFS or duration of response was 3.7 years (range, 0.1-8.6), with no significant difference according to subtypes: 3.71 years (range, 0.1-8) for indolent mastocytosis, 2.47 years (range, 0.5-8.6) for ASM, and 4.77 years (range, 0.3-6.4) for SM-AHNMD (Figure 2B).
Relapse-free survival (RFS) curves (A) and RFS curves according to mastocytosis subtypes (B); overall survival (OS) of the 68 patients treated by 2-CdA (C) and OS according to mastocytosis subtypes (D). ASM, aggressive systemic mastocytosis; AHNMD, associated hematologic non–mast cell disease; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; SSM, smoldering systemic mastocytosis.
Relapse-free survival (RFS) curves (A) and RFS curves according to mastocytosis subtypes (B); overall survival (OS) of the 68 patients treated by 2-CdA (C) and OS according to mastocytosis subtypes (D). ASM, aggressive systemic mastocytosis; AHNMD, associated hematologic non–mast cell disease; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; SSM, smoldering systemic mastocytosis.
After univariate analysis, factors significantly associated with a shorter RFS were: >4 courses of 2-CdA (HR 2.79 [range, 1.3-6.02]; P = .009) and a PR (HR 2.16 [range, 1.05-4.44]; P = .03) (Table 3).
Factors associated with RFS in 49 patients with mastocytosis treated by 2-CdA
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Male gender | 0.69 | 0.34-1.42 | .31 |
| AHNMD vs ISM/SSM/CM/MIS | 0.66 | 0.25-1.73 | .40 |
| ASM vs ISM/SSM/CM/MIS | 1.20 | 0.46-3.18 | .71 |
| Age at mastocytosis diagnosis >50 y | 1.25 | 0.62-2.52 | .53 |
| Age at 2-CdA initiation >55 y | 0.73 | 0.36-1.49 | .39 |
| Fatigue | 0.84 | 0.38-1.86 | .66 |
| Flush | 1.22 | 0.61-2.46 | .58 |
| Pruritus | 1.61 | 0.79-3.29 | .19 |
| Abdominal pain | 1.82 | 0.91-3.63 | .09 |
| Diarrhea | 1.39 | 0.69-2.81 | .36 |
| Hepatosplenomegaly | 1.06 | 0.53-2.09 | .88 |
| Urticaria pigmentosa | 1.42 | 0.70-0.55 | .46 |
| Bone involvement (yes vs no) | 0.50 | 0.24-1.04 | .06 |
| Anemia <10g/dL | 1.18 | 0.49-2.88 | .71 |
| >3 courses of 2-CdA | 1.72 | 0.84-3.49 | .14 |
| >4 courses of 2-CdA | 2.79 | 1.30-6.02 | .009 |
| Partial response | 2.16 | 1.05-4.44 | .037 |
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Male gender | 0.69 | 0.34-1.42 | .31 |
| AHNMD vs ISM/SSM/CM/MIS | 0.66 | 0.25-1.73 | .40 |
| ASM vs ISM/SSM/CM/MIS | 1.20 | 0.46-3.18 | .71 |
| Age at mastocytosis diagnosis >50 y | 1.25 | 0.62-2.52 | .53 |
| Age at 2-CdA initiation >55 y | 0.73 | 0.36-1.49 | .39 |
| Fatigue | 0.84 | 0.38-1.86 | .66 |
| Flush | 1.22 | 0.61-2.46 | .58 |
| Pruritus | 1.61 | 0.79-3.29 | .19 |
| Abdominal pain | 1.82 | 0.91-3.63 | .09 |
| Diarrhea | 1.39 | 0.69-2.81 | .36 |
| Hepatosplenomegaly | 1.06 | 0.53-2.09 | .88 |
| Urticaria pigmentosa | 1.42 | 0.70-0.55 | .46 |
| Bone involvement (yes vs no) | 0.50 | 0.24-1.04 | .06 |
| Anemia <10g/dL | 1.18 | 0.49-2.88 | .71 |
| >3 courses of 2-CdA | 1.72 | 0.84-3.49 | .14 |
| >4 courses of 2-CdA | 2.79 | 1.30-6.02 | .009 |
| Partial response | 2.16 | 1.05-4.44 | .037 |
AHNMD, associated hematologic non–mast cell disease; ASM, aggressive systemic mastocytosis; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; MIS, mastocytosis in the skin; SSM, smoldering systemic mastocytosis
Bold indicates significant parameters in univariate analysis.
Retreatment with additional courses of 2-CdA were given for 10 relapsed patients after a median time of response of 1.84 (range, 0.78-5.96) years. The median number of extra courses was 2 (range, 1-6). Among the 10 former responders, 9 patients responded again (8 MR, 1 PR).
Overall survival.
The overall survival rate was 48.5% (95% CI, 30.2-77.9) at the end of follow-up because 21 patients died (31%). Median survival time was 8.2 years (range, 21 d-9.1 y) (Figure 2C). The indolent subtypes were associated with a longer survival than the advanced subtypes (Figure 2D). In addition, 9 other factors were significantly associated with a poor prognosis (Table 4). After multivariate analysis, only mastocytosis subtypes (AHNMD vs indolent subtypes: HR = 6.59 [range, 1.32-32.88], P= .02) and ASM vs indolent subtypes (HR = 9.47 [range, 1.92-46.60], P= .006), and age at mastocytosis diagnosis >50 years (HR = 3.42 [range, 1.07-10.87, P= .038) were independently associated with mortality.
Factors associated with overall survival in 68 patients with mastocytosis treated by 2-CdA
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Male gender | 3.69 | 1.42-9.54 | .007 |
| AHNMD vs ISM/SSM/CM/MIS | 11.56 | 2.49-53.63 | .001* |
| ASM vs ISM/SSM/CM/MIS | 15.43 | 3.34-71.18 | .001* |
| Age at mastocytosis diagnosis >50 y | 7.26 | 2.41-21.88 | .001* |
| Age at 2-CdA initiation >55 y | 5.67 | 1.90-16.9 | .02 |
| Fatigue | 4.89 | 0.65-36.5 | .12 |
| Flush | 0.41 | 0.17-0.99 | .049 |
| Pruritus | 0.35 | 0.13-0.9 | .029 |
| Abdominal pain | 1.22 | 0.51-2.89 | .65 |
| Diarrhea | 0.82 | 0.34-1.96 | .66 |
| Hepatosplenomegaly | 16.45 | 2.2-122.9 | .006 |
| Urticaria pigmentosa | 0.16 | 0.07-0.39 | .0005 |
| Bone involvement | 1.52 | 0.64-3.61 | .35 |
| Anemia | 4.43 | 1.80-10.9 | .001 |
| Covariates . | HR . | 95% CI . | P . |
|---|---|---|---|
| Male gender | 3.69 | 1.42-9.54 | .007 |
| AHNMD vs ISM/SSM/CM/MIS | 11.56 | 2.49-53.63 | .001* |
| ASM vs ISM/SSM/CM/MIS | 15.43 | 3.34-71.18 | .001* |
| Age at mastocytosis diagnosis >50 y | 7.26 | 2.41-21.88 | .001* |
| Age at 2-CdA initiation >55 y | 5.67 | 1.90-16.9 | .02 |
| Fatigue | 4.89 | 0.65-36.5 | .12 |
| Flush | 0.41 | 0.17-0.99 | .049 |
| Pruritus | 0.35 | 0.13-0.9 | .029 |
| Abdominal pain | 1.22 | 0.51-2.89 | .65 |
| Diarrhea | 0.82 | 0.34-1.96 | .66 |
| Hepatosplenomegaly | 16.45 | 2.2-122.9 | .006 |
| Urticaria pigmentosa | 0.16 | 0.07-0.39 | .0005 |
| Bone involvement | 1.52 | 0.64-3.61 | .35 |
| Anemia | 4.43 | 1.80-10.9 | .001 |
AHNMD, associated hematological non–mast cell disease; ASM, aggressive systemic mastocytosis; CM, cutaneous mastocytosis; ISM, indolent systemic mastocytosis; MIS, mastocytosis in the skin; SSM, smoldering systemic mastocytosis.
Bold indicates significant parameters in univariate analysis.
Significant parameters after multivariate analysis.
Causes of death.
No case of death was suspected to be directly related to 2-CdA. Deaths related to mastocytosis progression (43%) according to mastocytosis subtypes were: 4 cases among 10 ASM patients (19%) and 5 cases among 9 AHNMD patients, including 2 cases with acute myeloid leukemia (AML) (24%). No death was a result of the progression of indolent to aggressive mastocytosis. Deaths not related to mastocytosis progression (57%) were: 5 cases of multi-organ failure, 2 of AML in ASM-AHNMD patients, probably linked to the AHNMD counterpart (1 with myeloproliferative neoplasm [primary myelofibrosis] and 1 with MDS [refractory anemia with excess of blasts]), 2 of solid tumors, 1 of acute respiratory distress syndrome, 1 of hemothorax, and 1 of septic shock.
Safety.
The clinical tolerance of 2-CdA treatment was good for most patients. Grade 3 or 4 toxicity was mostly related to myelosuppression and infectious causes.
Myelosuppression-related toxicity.
Thirty-two of 68 (47%) patients developed grade 3 (9; 13%)/grade 4 (23; 34%) acute neutropenia after at least one course of 2-CdA, and 56 of 68 (82%) patients developed grade 3-4 prolonged lymphopenia. Of note, 1 ISM patient who received 2-CdA monthly experienced a pancytopenia after 9 courses, requiring allogeneic hematopoietic stem cell transplantation with cure of mastocytosis.
Infectious toxicity.
Fifteen patients (22%) experienced suspected or confirmed infectious complications during 2-CdA administration. They consisted of 7 episodes of bacteremia (Staphylococcus epidermidis and Staphylococcus aureus, Escherichia coli, Corynebacterium spp, Pseudomonas aeruginosa, respectively, isolated for 3, 2, 1, and 1 patient), 1 Candida albicans fungemia, and 3 opportunistic infections (cutaneous monometameric Herpes zoster of the limb, cytomegalovirus viremia, and esophageal candidiasis in one patient each). Four patients experienced a fever of unknown origin despite exhaustive microbiological analyses. Late toxicity occurring beyond 3 months after the last 2-CdA administration course occurred in 6 patients (9%) with opportunistic infections (Pseudomonas aeruginosa pneumonia and Acinetobacter baumannii sepsis, Pseudomonas aeruginosa sepsis, Salmonella typhimurium sepsis, Streptococcus pneumoniae pneumonia and Staphylococcus hominis sepsis, Proteus vulgaris septic shock, and cutaneous monometameric Herpes zoster of the back in one case each. Altogether, 8 patients recovered from these severe infections and one died (septic shock). Severe toxicities were more frequently reported in ASM than in ISM (P < .01). Finally, no ISM patient died of toxicity related to 2-CdA.
Second solid malignancies.
Two solid tumors were observed during follow-up: 1 case of mucoepidermoïd parotid carcinoma and 1 case of glioblastoma, 4 years (9 courses) and 6.5 years (1 course) after the end of therapy, respectively.
Fertility.
After 2-CdA exposure, 2 women gave birth to normal children after an uneventful pregnancy without evidence of active disease. One became pregnant 18 months after 4 courses of 2-CdA for ISM at the age of 35 years. The other became pregnant 24 months after 6 courses of 2-CdA for ISM at the age of 39 years.
Discussion
In this nationwide retrospective study over a decade, our findings provide evidence that 2-CdA is an effective treatment of mastocytosis with an OR rate of 72%. The OR rate with 2-CdA varies from 55% to 100% in cases and series according to indolent or advanced M categories,3,11,14-16 compared with interferon-α (53%) and PKC412 (60%-68%) (supplemental Table 1).8,10
However, in contrast to previous studies,3,14 this therapy appeared more efficient in indolent mastocytosis (ISM-SSM and CM) patients than in ASM or ASM-AHNMD patients. The efficacy was evidenced for mediator release symptoms as well as in infiltration-related symptoms especially for those involving skin, gut, and fatigue. We confirmed previous limited reports of skin improvement (fading urticaria pigmentosa) with 2-CdA.14,23 Efficacy on biological parameters was not major except for tryptase concentrations over time, which reflects mast cell burden, mostly reduced in indolent mastocytosis. The IV route of administration of 2-CdA was more frequently used for hospitalized advanced mastocytosis patients than for indolent mastocytosis patients but with similar efficacy compared with subcutaneous administration of 2-CdA, allowing this route, which is more convenient for the outpatient care setting. Our study is the first with a long follow-up to evaluate the median time of 3.7 years (range, 0.1-8.6; 95% CI, 1.6-5.3) of duration of response to 2-CdA. Compared with interferon-α and PKC412 treatments that exhibit median durations of response of 12 and 24 months, respectively, and despite heterogeneous criteria of response, 2-CdA has a longer duration of response, but this comparison should be considered cautiously because our population included less advanced cases than in reports detailing interferon-α and PKC412. We observed that the median scheme of 2-CdA infusion was 4 courses, fewer than the 6 courses administrated to the single patient reported by Tefferi et al,16 and also to the 10 patients reported by Kluin-Nelemans et al14 in 2003. The length of the interval between courses, which varied between 6 weeks and 6 months, might also affect the efficacy, safety, and duration of responses in these case reports and series.
According to different SM variants, overall survival as expected from findings of other series was significantly longer for ISM patients than ASM and ASM-AHNMD patients.24 Our series with a median follow-up of 5.8 years (range, 21 d-9.1 y) reported 21 deaths. None of them was suspected to be related to 2-CdA treatment. We particularly focused on secondary malignancies and infections because 2-CdA has been previously reported to be associated with secondary malignancies because of its immunosuppressive function and DNA-damaging properties.25 In addition, the persistent lymphopenia has been considered a key factor in secondary malignancies, as observed in patients with hairy cell leukemia treated with 2-CdA. Two acute leukemia cases were observed in ASM patients. Because AML is known to occur in mastocytosis, and because of the low number of patients, it was difficult to link these 2 cases to 2-CdA treatment. Two solid tumors that were observed in our 2 patients were not listed in the series on long-term outcome of young patients with HCL,26 which reported the nonsignificant excess frequency of secondary malignancies with 2-CdA treatment. Taken together, our data suggest that 2-CdA was not associated with the development of secondary tumors in mastocytosis.
In addition to risks of secondary malignancies, the main toxicities in our series were infectious and related to myelosuppression. They were infrequent and affected mostly ASM and AHNMD patients with initial cytopenia. 2-CdA has been shown to induce prolonged B-cell and T-cell lymphopenia, in particular with a profound suppression of the CD4+ T-cell subset for at least almost 2 years’ duration,27 a justification for giving long-term antiinfective prophylaxes.
Although our findings are based on a low number of patients, it should also be stressed that 2-CdA did not seem to impair female fertility. However, because of this small number of patients, 2-CdA should still be used more cautiously in women of child-bearing age. This point is of interest because this purine analog was thought to have consequences on fertility because of DNA strand breaks. In HCL, 2-CdA treatment was also not associated with infertility.28
In this study with inherent limitations for definite conclusions because of its retrospective design, we might consider secondarily a lower dose or modified scheme of administration of 2-CdA for indolent mastocytosis patients to improve the benefit-risk balance. As observed in some advanced mastocytosis patients who relapsed after major responses after 2-CdA treatment, one could expect a synergistic effect of drug combination between PKC412 and/or other tyrosine kinase inhibitors and 2-CdA to induce a clinical response.8,10 To avoid relapse, maintenance therapy might be a challenge to consider to stabilize mast cell release mediators in ISM and CM patients with careful monitoring of toxicity. The most appropriate time interval between 2-CdA treatment could be determined by a patient’s CD34– mast cell precursors analysis based on multiparameter flow cytometry as described by our group.29
In conclusion, 2-CdA is an effective treatment with an acceptable safety profile in mastocytosis patients refractory to multiple symptomatic therapies or in those with advanced presentations, and might be considered as a treatment option in symptomatic CM or ISM patients in consideration of the benefit-risk balance. Further work is warranted to define the optimal regimen as well as clinical relevance of maintenance 2-CdA therapy during mastocytosis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all contributors for clinical support of CEREMAST competence network for mastocytosis from France.
This work was supported by grants from la Société Française de Dermatologie (S.F.D.) and CEREMAST (Centre de Référence des Mastocytoses), and by INSERM and la Ligue Nationale Contre le Cancer Équipe Labellisée (P.D.).
Authorship
Contribution: O.H., O.L., S.B., and I.H. conceived and designed the study; O.H. and I.H. provided administrative support; S.B., I.H., O.H., O.L., C.E., M.O.C., and G.D. collected and assembled data; S.B., O.H., O.L., C.E., I.H., G.D., M.O.C., and E.G. analyzed and interpreted data; C.E. gathered statistics; and all authors provided study materials or patients, contributed to writing the paper, and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphane Barete, Department of Dermatology and Allergy, Hôpital Tenon, 4 rue de la Chine, Paris 75020, France; e-mail: stephane.barete@psl.aphp.fr.