Key Points
Prenatal platelet-forming lineages are subject to common transcription factor controls despite distinct spatial and ancestral origins.
Platelet-forming lineage production is MPL-independent on emergence, but MPL is required in the late fetus for efficient thrombopoiesis.
Abstract
The thrombopoietic environment of the neonate is established during prenatal life; therefore, a comprehensive understanding of platelet-forming cell development during embryogenesis is critical to understanding the etiology of early-onset thrombocytopenia. The recent discovery that the first platelet-forming cells of the conceptus are not megakaryocytes (MKs) but diploid platelet-forming cells (DPFCs) revealed a previously unappreciated complexity in thrombopoiesis. This raises important questions, including the following. When do conventional MKs appear? Do pathogenic genetic lesions of adult MKs affect DPFCs? What role does myeloproliferative leukemia virus (MPL), a key regulator of adult megakaryopoiesis, play in prenatal platelet-forming lineages? We performed a comprehensive study to determine the spatial and temporal appearance of prenatal platelet-forming lineages. We demonstrate that DPFCs originate in the yolk sac and then rapidly migrate to other extra- and intraembryonic tissues. Using gene disruption models of Gata1 and Nfe2, we demonstrate that perturbing essential adult MK genes causes an analogous phenotype in the early embryo before the onset of hematopoietic stem/progenitor cell-driven (definitive) hematopoiesis. Finally, we present the surprising finding that DPFC and MK commitment from their respective precursors is MPL independent in vivo but that completion of MK differentiation and establishment of the prenatal platelet mass is dependent on MPL expression.
Introduction
In humans, early-onset thrombocytopenia (TP) can be present at birth or emerge progressively over early life.1,2 Studies of both human patients and mouse knockout models have revealed that multiple molecular dysfunctions can underlie TP; for example, mutation of myeloproliferative leukemia virus (MPL) drives analogous congenital amegakaryocytic thrombocytopenia (CAMT) in humans3-5 and mice6-14 ; disruption of Gata1 expression causes X-linked thrombocytopenia in human males15-17 and megakaryocyte (MK) hyperproliferation with associated thrombocytopenia in mice18 ; and mutation of Runx1 underlies human familial platelet disorder19-23 and in mice causes megakaryocytopenia and thrombocytopenia.24-26 It is likely that differences in timing of TP onset are causally related to which prenatal phase of hematopoiesis is affected (primitive vs definitive). Accordingly, it is critical to develop a clear understanding of the formation of the prenatal platelet-forming system.
Mouse models of hematopoietic development have proved instrumental in elucidating pre/postnatal thrombopoiesis. Elegant studies of early hematopoiesis revealed that conventional (highly polyploid) megakaryocytic differentiation potential is one of the first adult-like traits to emerge, appearing along the primitive erythroid lineage.27 We recently described that despite having the potential to generate highly polyploid MKs, the first platelet-forming lineage of the conceptus appears in the extraembryonic yolk sac (YS) as cells that produce platelets while in a diploid state.28 Termed diploid platelet-forming cells (DPFCs), these cells are specified via a progenitor-independent hematopoietic pathway. Later in development, hematopoietic stem/progenitor cell (HSPC)-derived conventional MKs appear in the liver, which engage in extensive platelet formation to establish adequate thrombocytic capacity for the postnatal organism.29
The discovery of a biphasic origin of prenatal thombopoiesis raises important questions. (1) what are the temporal and spatial kinetics of HSPC-derived megakaryopoiesis? (2) Are DPFCs and MKs subject to distinct or overlapping molecular regulatory controls? (3) When do neonatal/adult TP phenotypes emerge? Answers to these questions are not only important to understanding physiologic hematopoiesis, but will also facilitate greater understanding about when pathology-associated genetic lesions begin to exert their influence.
The actions of thrombopoietin (THPO) via MPL are thought to be critical for the successive development of the MK lineage from multipotent precursors and for effective platelet production both in vitro and in vivo.6-14 These mouse-based observations are consistent with the megakaryocytic and thrombocytic components of the human CAMT disorder,30 where disease severity correlates with degree of failure of THPO/MPL signaling.5,31,32 However, an outstanding inconsistency was that, although CAMT patients can present with TP as neonates,5,31 the disease could also progress within the first year of life.5,31 Accordingly, it is timely for the etiology of CAMT-like TP to be experimentally revisited.
In this study, we used advanced microscopy, in vitro functional assays, and flow cytometry to comprehensively map the kinetics of both prenatal platelet-forming lineages (DPFCs and MKs) and the establishment of robust platelet production. We herein show that the embryonic day (E)8.5 YS is the source of the first platelet-forming cells, which rapidly colonize the E9.5 placenta and embryo proper. We demonstrate that, although the hematopoietic progenitor compartment of the YS initiates MK differentiation in situ, terminal differentiation is accomplished in the liver, resulting in the formation of MKs up to 32n by E16.5, representing a supersession of platelet production by DPFCs to MK derived. We show that, although differences in transcription factor (TF) expression exist between YS DPFCs and liver MKs, core TFs of the conventional MK lineage are expressed and functionally required by DPFCs. To exemplify this point, we reveal that disruption of Gata1 expression with the platelet-forming lineages imposes an early hyperproliferation phenotype that precedes stem/progenitor-derived hematopoiesis.
We present the surprising finding that commitment of both platelet-forming lineages from their respective precursors is completely MPL independent in vivo. Finally, we identified a window of time during the life of the organism when MPL is required within the MK lineage: failure of MPL signaling during fetal life results in a MK differentiation block at the 8n stage, imposing an inadequacy in the expansion of the late fetal platelet mass.
Together, these findings provide a framework within which the causative cellular mechanisms underlying the timing of onset of postnatal TP, such as CAMT, can be interpreted.
Methods
Mice
UBI-gfp,33 Gata1ΔHS,18 Mpl−/−,7 and Nfe2−/−34 mouse lines were on a C57BL/6 background. Experimental procedures were approved by the Walter and Eliza Hall Institute Animal Ethics Committee. Developmental stages were determined by somite pair numbers (E8.0-E9.5) or morphologically by Theiler’s criteria (≥E10.5).
Flow cytometry
Tissues were incubated in 10% collagenase-dispase (Roche) for 45 to 60 minutes at 37°C and then mechanically dissociated. Cells were stained using combinations of anti-VE-CADHERIN (BV13), -CD45 (30-F11), -CD41 (MWreg30), -CD42D (Gon.C2), and -MPL (AMM2). 7-Aminoactinomycin D (7-AAD) was used for viability assessment. Peripheral blood was collected from individual embryos in EDTA/7% fetal calf serum/calcium and magnesium–free Dulbecco's phosphate-buffered saline. For ploidy analysis, stained cells were ethanol fixed and stained with 4,6 diamidino-2-phenylindole/0.1% Triton X-100. Flow cytometry was performed using a BD LSRFortessa or BD FACSAria. A 95% sort purity cutoff was imposed.
Microarray analysis
Previously published E10.5 YS DPFC and E13.5 liver MK expression data (ArrayExpress; E-MTAB-262528 ) were analyzed using limma software.35 Intensity values were normexp background corrected and quantile normalized using the neqc function.36 Perfect/good probes37 that achieved a detection P < .05 on at least half of the arrays were used. Where multiple probes were assigned to a single gene identifier, the probe with the highest mean expression was selected for gene-level analyses. Differential expression was assessed using empirical Bayes moderated t statistics.38 Genes with a false discovery rate < 0.05 were considered differentially expressed.
Whole-mount confocal microscopy
Samples were fixed in 2% paraformaldehyde with 0.1% Tween-20, and blocked/permeabilized in 10% fetal calf serum/DPBS with 0.6% Triton X-100. Antibody staining was performed either overnight at 4°C or 6 to 8 hours at room temperature using combinations of: anti-CD41, -CD42D, and -VE-CADHERIN. Placentas and livers were cleared in a glycerol gradient.39 Whole embryos were cleared using tetrahydrofuran and dibenzyl ether.40 Images were acquired on a Zeiss LSM 780 and analyzed using Imaris (Bitplane).
Colony-forming assays
For megakaryocyte colony forming unit (CFU-MK), MegaCult-C (StemCell Technologies) was used as previously described.28 After 5 days, cultures were PFA fixed, acetone dehydrated, stained to detect CD41 expression using Vectastain Elite ABC kit, and counterstained with methyl green to detect nuclei. Cultures were scored manually, and colonies were defined as clusters of >10 CD41+ cells.28 M3434 (StemCell Technologies) was used for myelo-erythroid assays according to the manufacturer’s instructions. Colonies were scored after 7 days.41
Proplatelet-forming assay
Purified cells were cultured in defined serum-free medium (SFM)42 with 100 ng/mL recombinant murine THPO (Peprotech) and anti-CD41-APC and anti-CD42D-PE antibodies in 8-well coverslip chamber slides (Ibidi). Live cultures were imaged on a Deltavision Elite microscope before harvesting for ploidy analysis. For THPO dependency experiments, 1000 to 2000 purified E10.5 YS CD45−CD41high DPFCs and CD45+CD41low HPCs were cultured with or without 100 ng/mL THPO. Following culture, cells were gently harvested, 7AAD was added, and the entire sample analyzed by flow cytometry to quantify CD41highCD42D+ cells.
Results
Diploid platelet-forming cells arise first in the E8.5 YS and then rapidly migrate to intra- and extraembryonic tissues
The foundation of MK and platelet disorders of early life is inextricably linked to hematopoiesis in prenatal life. To generate a conceptus-wide overview of how thrombopoiesis is established, we undertook a broad investigation of the temporal and spatial pattern of platelet-forming lineage emergence within the early embryo, encompassing both DPFCs28 and conventional (highly polyploid) MKs.
Although it is generally accepted that the first hematopoietic cells of the E8.5 embryo proper (EP) are migrants of YS origin,43 cumulating evidence suggests that multiple autonomous sites of de novo hematopoiesis exist in the conceptus.44-46 With regard to the platelet-forming lineages, studies to date have focused entirely on detecting mature cells within the YS; thus, the potential contributions of other hematogenic organs of the conceptus remain uninvestigated.
We observed that at E8.5 (5-10 somite pairs), CD41highGATA1+RUNX1+ hematopoietic cells were distributed within vascularized regions of the YS, EP, and within clusters of the post-chorioallantoic fusion placenta (supplemental Figure 1A-C available on the Blood Web site). Flow cytometric analysis revealed that very few cells with the CD41highCD42D+ DPFC immunophenotype28 were present at E8.5 (supplemental Figure 1D). By E9.5, CD41highCD42D+ cells concomitantly appeared in the YS, EP, placenta, and peripheral blood (Figure 1A). Taking advantage of advanced confocal microscopy and tissue clearing methods, we were able to identify that the first DPFCs of the embryo were found within the head vasculature, splanchnic mesenchyme, occasionally within the mesenchyme of the developing liver bud, and within the placental vasculature (Figure 1B; supplemental Figure 2).
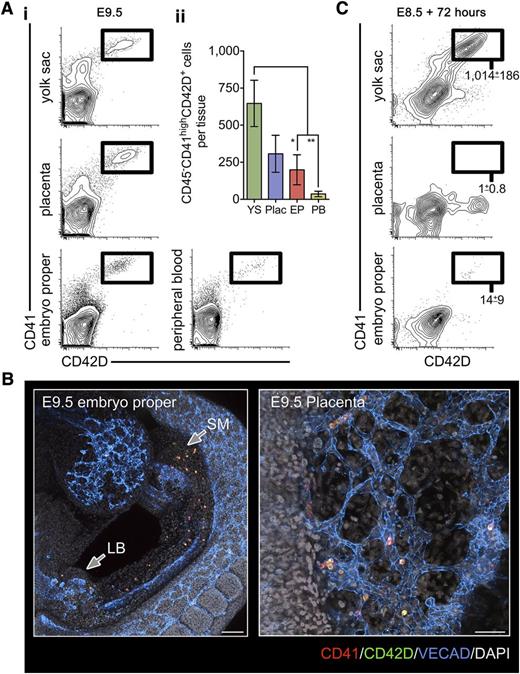
The early YS is the source of the first platelet forming cells in the early conceptus. (A) (i) Representative flow cytometry plots of the E9.5 YS, placenta, EP, and peripheral blood stained with markers for DPFCs: CD41 and CD42D. (ii) Quantification of absolute numbers of CD41highCD42D+ DPFCs in the E9.5 conceptus, demonstrating a significant enrichment of cells in the YS and placenta (Plac). *P < .05, **P < .01 (n = 3 independent experiments, using 13 individual embryos). Bars represent standard deviation. (B) Representative 3-dimensional projection of a confocal z-stack showing the distribution of CD41 (red) and CD42D (green) coexpressing cells in the embryo proper (bar, 200 μm) and placenta (bar, 50 μm) at E9.5 (n = 9). VE-CADHERIN (VECAD) labels the vasculature (blue), and 4,6 diamidino-2-phenylindole (gray) was used to stain nuclei. (C) Representative plots from culture of 2 embryo equivalents of E8.5 (5-10 somite pairs) YS, placenta, and EP cell suspensions after 72 hours in proplatelet-forming conditions. Values represent the mean ± standard error of the mean (SEM) of CD41highCD42D+ cells produced per embryo equivalent of input cells (n = 3).
The early YS is the source of the first platelet forming cells in the early conceptus. (A) (i) Representative flow cytometry plots of the E9.5 YS, placenta, EP, and peripheral blood stained with markers for DPFCs: CD41 and CD42D. (ii) Quantification of absolute numbers of CD41highCD42D+ DPFCs in the E9.5 conceptus, demonstrating a significant enrichment of cells in the YS and placenta (Plac). *P < .05, **P < .01 (n = 3 independent experiments, using 13 individual embryos). Bars represent standard deviation. (B) Representative 3-dimensional projection of a confocal z-stack showing the distribution of CD41 (red) and CD42D (green) coexpressing cells in the embryo proper (bar, 200 μm) and placenta (bar, 50 μm) at E9.5 (n = 9). VE-CADHERIN (VECAD) labels the vasculature (blue), and 4,6 diamidino-2-phenylindole (gray) was used to stain nuclei. (C) Representative plots from culture of 2 embryo equivalents of E8.5 (5-10 somite pairs) YS, placenta, and EP cell suspensions after 72 hours in proplatelet-forming conditions. Values represent the mean ± standard error of the mean (SEM) of CD41highCD42D+ cells produced per embryo equivalent of input cells (n = 3).
To assess whether YS, EP, and placenta were each autonomously capable of DPFC formation or whether DPFCs present at E9.5 were immigrants of YS origin, E8.5 single cell preparations from each region were cultured in serum-free medium supplemented with THPO (conditions that enable production of CD41highCD42D+ cells and the induction of proplatelet formation from E8.5 YS28 ). Only E8.5 YS cultures were capable of generating large numbers of CD41highCD42D+ cells (Figure 1C). These data indicate that DPFCs observed in the E9.5 conceptus were likely of YS origin that had been disseminated via the peripheral circulation.
Despite extensive thrombopoiesis, highly polyploid megakaryocytes are not present in the E10.5 conceptus
By E10.5 (30-35 sp) CD41highCD42D+ cells were distributed throughout the conceptus (Figure 2A). CD41highCD42D+ cells and platelets were readily detected in the liver and placenta and within the peripheral circulation (Figure 2A-B). Of note, the origin of placental CD41highCD42D+ cells was confirmed as embryonic (supplemental Figure 3). In keeping with the E10.5 YS,28 highly polyploid MKs were not detected in any of the tissues analyzed (Figure 2C; supplemental Figure 4), indicating that all platelet production was DPFC derived as conventional MKs had not yet emerged.
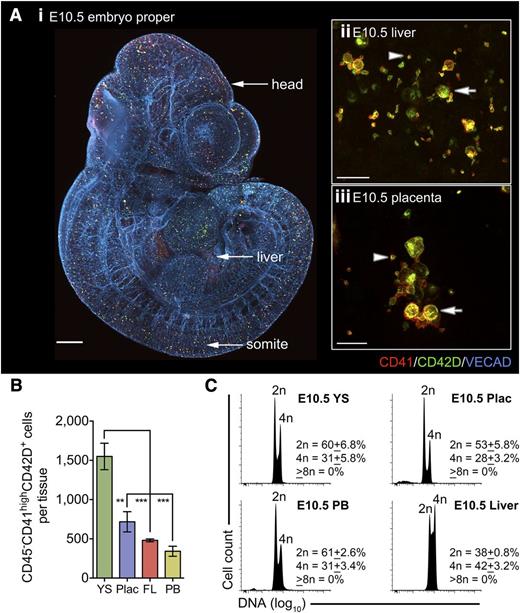
DPFCs, but not highly polyploid megakaryocytes, are distributed across the entire conceptus by E10.5. (A) (i) Representative 3-dimensional projection of confocal z-stacks from a chemically cleared E10.5 embryo. CD41+CD42D+ cells are observed throughout vascularized regions, including the head and somites (n = 10; bar, 200 μm). (ii-iii) Representative 3-dimensional projection of confocal z-stacks from glycerol-cleared organs revealing the presence of CD41+CD42D+ cells (arrows) and platelets (arrowheads) in the E10.5 (ii) liver and (iii) placenta. Bars, 20 μm. (B) Quantification of absolute numbers of CD41highCD42D+ cells in key hematogenic organs of the E10.5 conceptus reveals a significant enrichment in the YS despite the onset of circulation. Data are cumulative of 3 independent experiments, **P < .01; ***P < .001. (C) DNA content analysis on pooled embryonic samples demonstrating that all CD41highCD42D+ cells in the YS, peripheral blood (PB), placenta (Plac), and liver are low ploidy (2n-4n), with the majority in a diploid state. Those in the liver had a greater proportion of cells in S-phase and 4n. (n = 3).
DPFCs, but not highly polyploid megakaryocytes, are distributed across the entire conceptus by E10.5. (A) (i) Representative 3-dimensional projection of confocal z-stacks from a chemically cleared E10.5 embryo. CD41+CD42D+ cells are observed throughout vascularized regions, including the head and somites (n = 10; bar, 200 μm). (ii-iii) Representative 3-dimensional projection of confocal z-stacks from glycerol-cleared organs revealing the presence of CD41+CD42D+ cells (arrows) and platelets (arrowheads) in the E10.5 (ii) liver and (iii) placenta. Bars, 20 μm. (B) Quantification of absolute numbers of CD41highCD42D+ cells in key hematogenic organs of the E10.5 conceptus reveals a significant enrichment in the YS despite the onset of circulation. Data are cumulative of 3 independent experiments, **P < .01; ***P < .001. (C) DNA content analysis on pooled embryonic samples demonstrating that all CD41highCD42D+ cells in the YS, peripheral blood (PB), placenta (Plac), and liver are low ploidy (2n-4n), with the majority in a diploid state. Those in the liver had a greater proportion of cells in S-phase and 4n. (n = 3).
YS hematopoietic progenitor cells begin megakaryocytic differentiation in situ but do not become highly polyploid until after liver engraftment
As early as E8.5, YS hematopoietic progenitor cells (HPCs) have the potential to generate highly polyploid MKs following ex vivo culture,27,28,47 yet it is not known whether MK differentiation from HPCs is initiated in the YS in vivo.
We previously observed that maturation of E8.5 YS pre-DPFCs was accompanied by an increase in CD42D expression.28 Based on this observation, we explored whether CD42D could be used as a putative early marker of MK differentiation from YS HPCs (defined by a CD45+CD41low immunophenotype). Between E9.5 and E10.5, we found that a fraction of YS HPCs had initiated CD42D expression (Figure 3A). When E10.5 YS HPCs were purified into CD42D+ and CD42D− counterparts and subjected to in vitro functional assays, we found that clonogenic potential was greater among CD42D− HPCs (Figure 3B). Both CD42D+ and CD42D− HPCs generated polyploid MKs after 72 hours in vitro (Figure 3Ci); however, acute proplatelet formation (a property of mature platelet-forming cells28,48 ) was only observed from CD42D+ HPCs (Figure 3Cii). Consistent with reports of MK differentiation from fetal liver stem/progenitor cells,49 CD42D− HPCs required a total of 6 days of culture before proplatelet formation was observed (data not shown).
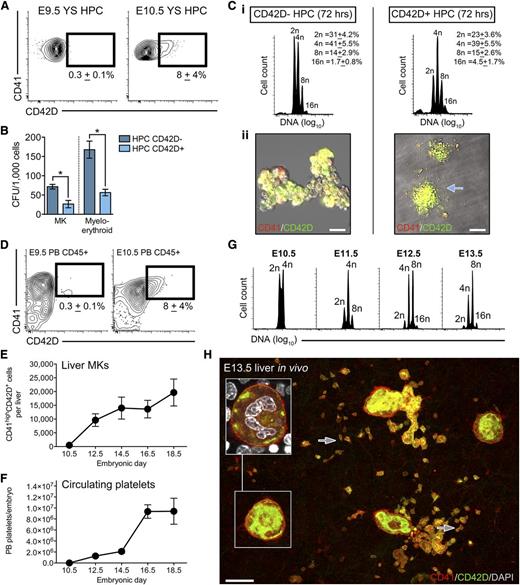
Defining the onset of progenitor-derived megakaryopoiesis in vivo. (A) Representative flow cytometry plots of E9.5 and E10.5 YS CD45+CD41low HPC populations showing the acquisition of CD42D expression by a subpopulation of cells at E10.5 (n = 9). (B) CFU-MK and CFU-myeloid/erythroid frequency (per 1000 cells plated) in E10.5 YS CD42D− and CD42D+ HPC populations. Bars, SEM (n = 3). *P < .05. (C) Purified E10.5 YS HPCs (CD45+CD41low cells), segregated according to CD42D expression, were cultured in proplatelet-forming medium for 72 hours. (i) Ploidy analysis by flow cytometry revealed that although highly polyploid MKs were generated by both fractions, only CD42D+ HPCs were capable of acute proplatelet formation (ii); n = 3. Arrow indicates proplatelet. (D) Representative flow cytometry plots of E9.5 and E10.5 peripheral blood (PB) demonstrating that CD42D+ HPCs entered the circulation from E10.5; n = 3. Values represent the number of CD45+CD41lowCD42D+ cells per embryo (±standard deviation). (E) Quantification of CD41highCD42D+ MKs in individual livers from E10.5 to E18.5 embryos, demonstrating that numbers dramatically increase until E14.5, from which point they remain relatively stable. n = 7 to 15 embryos per developmental stage. Bars, standard deviation. (F) Quantification of circulating platelet numbers in the peripheral blood of individual E10.5 to E18.5 embryos, revealing a gradual increase in numbers until E14.5, and a rapid spike by E16.5. n = 7 to 15 embryos per developmental stage. Bars, standard deviation. (G) Ploidy analysis of CD41highCD42D+ liver MKs from E10.5 to E13.5 reveals that the increase in circulating platelet numbers coincides with an increase ploidy of the liver MK population. Highly polyploid MKs are first seen at E11.5 (achieving an 8n state), from which point the population successively achieves higher ploidy states. n = 4 to 18 individual embryos per developmental stage. (H) Representative 3-dimensional projection of a confocal z-stack of CD41+ (red) and CD42D+ (green) MKs in the E13.5 liver. Inset is an optical section of boxed region showing a highly polyploid MK. Arrows indicated examples of platelets, which often display filopodia extensions. Bar, 20 μm. n = 11.
Defining the onset of progenitor-derived megakaryopoiesis in vivo. (A) Representative flow cytometry plots of E9.5 and E10.5 YS CD45+CD41low HPC populations showing the acquisition of CD42D expression by a subpopulation of cells at E10.5 (n = 9). (B) CFU-MK and CFU-myeloid/erythroid frequency (per 1000 cells plated) in E10.5 YS CD42D− and CD42D+ HPC populations. Bars, SEM (n = 3). *P < .05. (C) Purified E10.5 YS HPCs (CD45+CD41low cells), segregated according to CD42D expression, were cultured in proplatelet-forming medium for 72 hours. (i) Ploidy analysis by flow cytometry revealed that although highly polyploid MKs were generated by both fractions, only CD42D+ HPCs were capable of acute proplatelet formation (ii); n = 3. Arrow indicates proplatelet. (D) Representative flow cytometry plots of E9.5 and E10.5 peripheral blood (PB) demonstrating that CD42D+ HPCs entered the circulation from E10.5; n = 3. Values represent the number of CD45+CD41lowCD42D+ cells per embryo (±standard deviation). (E) Quantification of CD41highCD42D+ MKs in individual livers from E10.5 to E18.5 embryos, demonstrating that numbers dramatically increase until E14.5, from which point they remain relatively stable. n = 7 to 15 embryos per developmental stage. Bars, standard deviation. (F) Quantification of circulating platelet numbers in the peripheral blood of individual E10.5 to E18.5 embryos, revealing a gradual increase in numbers until E14.5, and a rapid spike by E16.5. n = 7 to 15 embryos per developmental stage. Bars, standard deviation. (G) Ploidy analysis of CD41highCD42D+ liver MKs from E10.5 to E13.5 reveals that the increase in circulating platelet numbers coincides with an increase ploidy of the liver MK population. Highly polyploid MKs are first seen at E11.5 (achieving an 8n state), from which point the population successively achieves higher ploidy states. n = 4 to 18 individual embryos per developmental stage. (H) Representative 3-dimensional projection of a confocal z-stack of CD41+ (red) and CD42D+ (green) MKs in the E13.5 liver. Inset is an optical section of boxed region showing a highly polyploid MK. Arrows indicated examples of platelets, which often display filopodia extensions. Bar, 20 μm. n = 11.
These data are consistent with a conventional differentiation model, where as HPCs exit from the progenitor state, clonogenic potential is gradually lost and the latency of MK maturation reduced,48,49 enabling acute proplatelet formation. Thus, in the E10.5 YS, a fraction of CD45+CD41low cells had initiated MK differentiation and could be isolated according to CD42D coexpression.
That highly polyploid MKs are not found in the YS or any other tissue of the E10.5 conceptus (Figure 2) suggests that initiation of megakaryopoiesis from HPCs can occur in the YS but is likely completed in secondary hematopoietic organs later in development. From E10.5, HPCs seed the liver, most of which are thought to migrate from the YS43 ; thus, it is likely that MKs formed in the liver over subsequent days derive from YS HPCs.
Consistent with the YS-to-EP seeding model, CD45+CD41lowCD42D+ cells were found transiting within the peripheral blood of E10.5 embryos (Figure 3D). The presence of circulating CD45+CD41lowCD42D+ cells coincided with the onset of megakaryopoiesis in the fetal liver, after which the liver MK population increased from ∼500 at E10.5 to 20 000 by E18.5 (Figure 3E). The increase in MK numbers was mirrored by a striking expansion in the number of circulating platelets, which increased from ∼1 × 104 at E10.5 to 1 × 107 by E18.5 (Figure 3F). This increase in platelet mass within the embryos was accompanied by the rapid appearance of highly polyploid liver MKs between E10.5 and E13.5, characterized by a progressive loss of 2n cells toward a 16n state (Figure 3G-H; supplemental Table 1).
Despite a number of differentially expressed transcription factors, expression of functionally important megakaryocyte genes are conserved in DPFCs
The embryonic platelet-forming lineages can therefore be categorized according to (1) respective precursor (primitive vs multipotent progenitor); (2) primary site of production (YS vs liver); and (3) genome size (diploid vs highly polyploid). This leads to a fundamental question: are genes essential for DPFCs and conventional MKs distinct or overlapping?
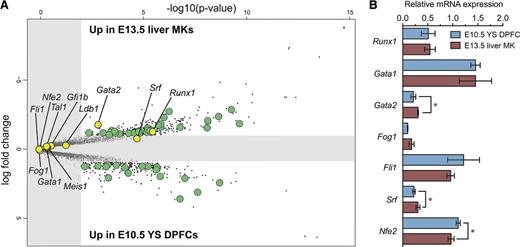
To address whether differences also extend to underlying regulatory programs, we analyzed previously published gene expression arrays for E10.5 YS DPFCs and E13.5 liver MKs,28 from which we focused on TFs. A number of TFs were differentially expressed between E10.5 YS DPFCs and E13.5 liver MKs (Figure 4A; supplemental Table 2A-B), but interestingly, of those TFs with known roles in fetal/adult megakaryopoiesis (Fli1,50 Gata1,18 Gata2,51 Gfi1b,52 Fog1,53 Ldb1,54 Meis1,55 Nfe2,34 Runx1,24 Srf,56 and Tal157 ), few were differentially expressed (Figure 4A; supplemental Table 2C). Of those tested by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), only minor differences in Gata2, Srf, and Nfe2 expression were detected (Figure 4B).
Comparative analysis of transcription factor expression between E10.5 YS DPFCs and conventional progenitor cell-derived E13.5 liver MKs. (A) Volcano plot comparing gene expression between E10.5 YS DPFCs and E13.5 liver MKs. Gray area indicates no significant expression difference. TFs are shown in green, and TFs of known functional importance in fetal or adult MKs are shown in yellow. (B) qRT-PCR validation of a cohort of functionally important MK TFs in YS and fetal liver MK samples. Data points are relative to Hmbs-1 expression and are expressed as mean ± SEM (n = 3). *P < .05.
Comparative analysis of transcription factor expression between E10.5 YS DPFCs and conventional progenitor cell-derived E13.5 liver MKs. (A) Volcano plot comparing gene expression between E10.5 YS DPFCs and E13.5 liver MKs. Gray area indicates no significant expression difference. TFs are shown in green, and TFs of known functional importance in fetal or adult MKs are shown in yellow. (B) qRT-PCR validation of a cohort of functionally important MK TFs in YS and fetal liver MK samples. Data points are relative to Hmbs-1 expression and are expressed as mean ± SEM (n = 3). *P < .05.
Loss of Gata1 and Nfe2 in YS DPFCs phenocopy conventional MKs
This raised the intriguing possibility that despite originating through distinct pathways and in different organs, a number of TFs are core to both platelet-forming lineages. To pursue this idea, we selected 2 TFs (Gata1 and Nfe2) for in vivo validation experiments based on their distinct roles: GATA1 is essential for formation of mature MKs, and disrupted expression in the MK lineage results in hyperproliferation in vitro and in vivo,18 whereas NFE2 is required for platelet formation.35
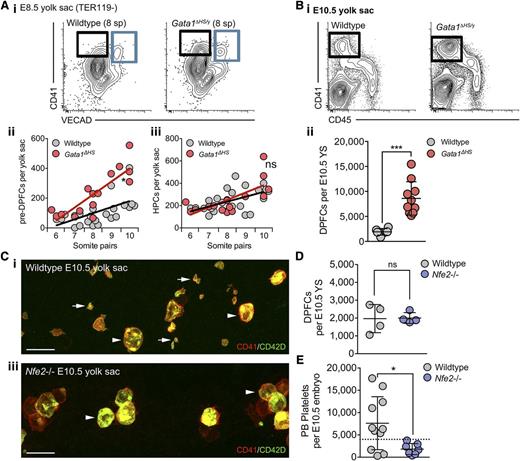
Consistent with CD41+VECAD− cells in the E8.5 YS being precursors to the DPFC lineage, Gata1ΔHS embryos exhibited an early increase in the number of pre-DPFCs cells from the 8 somite pair stage (Figure 5Ai-ii), whereas the mutation did not affect the HPC population (Figure 5Ai-iii). This gain of function of the YS DPFC lineage continued over subsequent days, resulting in a fourfold increase in DPFCs by E10.5 (Figure 5Bi-ii). Thus, perturbation of Gata1 expression results in an early manifestation of a hyperproliferative phenotype in the DPFC lineage.
GATA1 and NF-E2 operate within the DPFC lineage in a manner consistent with adult MKs. (A) (i) Representative flow cytometry plots from E8.5 age-matched (8 somite pairs) wild-type and Gata1ΔHS/y mutant YSs stained with anti-CD41 and -VECAD antibodies. Pre-DPFCs are gated in black, and the hematopoietic progenitor population is in blue. (ii-iii) Quantification of pre-DPFC and HPC numbers per YS at E8.5 according to discrete somite pair stages. Linear regression analysis revealed a DPFC lineage-specific phenotype, in which an early expansion in pre-DPFCs occurs from the 8 somite pair stage, whereas the HPC population remains quantitatively unaltered. ns, not significant, *P < .05. (B) (i) Representative flow cytometry plots and (ii) absolute quantification of E10.5 YS DPFCs numbers in individual wild-type and Gata1ΔHS mutant embryos. ***P < .0001. Bars, standard deviation. (C) Representative 3-dimensional projection of confocal z-stacks from (i) wild-type and (ii) Nfe2−/− E10.5 intact YSs stained with anti-CD41 (red) and -CD42D (green) antibodies. DPFCs are indicated by arrowheads and platelets by arrows. n = 13. (D-E) Quantification of (D) DPFC numbers in the YS and (E) platelets in the peripheral blood of E10.5 individual wild-type and Nfe2−/− embryos. n = 4 to 10; bar represents standard deviation. ns, not significant. *P < .05. Dashed line represents the upper limit of maternal platelet contamination to each sample, as previously described.28
GATA1 and NF-E2 operate within the DPFC lineage in a manner consistent with adult MKs. (A) (i) Representative flow cytometry plots from E8.5 age-matched (8 somite pairs) wild-type and Gata1ΔHS/y mutant YSs stained with anti-CD41 and -VECAD antibodies. Pre-DPFCs are gated in black, and the hematopoietic progenitor population is in blue. (ii-iii) Quantification of pre-DPFC and HPC numbers per YS at E8.5 according to discrete somite pair stages. Linear regression analysis revealed a DPFC lineage-specific phenotype, in which an early expansion in pre-DPFCs occurs from the 8 somite pair stage, whereas the HPC population remains quantitatively unaltered. ns, not significant, *P < .05. (B) (i) Representative flow cytometry plots and (ii) absolute quantification of E10.5 YS DPFCs numbers in individual wild-type and Gata1ΔHS mutant embryos. ***P < .0001. Bars, standard deviation. (C) Representative 3-dimensional projection of confocal z-stacks from (i) wild-type and (ii) Nfe2−/− E10.5 intact YSs stained with anti-CD41 (red) and -CD42D (green) antibodies. DPFCs are indicated by arrowheads and platelets by arrows. n = 13. (D-E) Quantification of (D) DPFC numbers in the YS and (E) platelets in the peripheral blood of E10.5 individual wild-type and Nfe2−/− embryos. n = 4 to 10; bar represents standard deviation. ns, not significant. *P < .05. Dashed line represents the upper limit of maternal platelet contamination to each sample, as previously described.28
Given that NFE2 is required for platelet formation, we investigated Nfe2−/− embryos at E10.5, the stage at which substantial numbers of platelets are first detected.27,28 Using 3-dimensional confocal microscopy of whole YS, we could readily identify DPFCs, but platelets were absent (Figure 5C). Quantification of the numbers of DPFCs in the E10.5 YS and platelet numbers in the peripheral blood confirmed that absence of NFE2 did not affect DPFC formation but prevented platelet formation (Figure 5D-E). Thus, as with conventional MKs in the fetus and the adult, Nfe2 expression is critical for platelet formation from DPFCs.
These data establish that at 2 key landmarks of platelet-forming lineage development, DPFCs are subject to similar regulatory controls as their highly polyploid counterparts.
Thrombopoiesis from DPFCs is MPL independent but progenitor cell-derived megakaryocytes require MPL to complete differentiation
Until recently, it was generally accepted that the cytokine THPO acting through MPL is instrumental to the successful production of mature MKs and effective platelet production. However, using in vivo conditional deletion strategies, Ng et al58 recently demonstrated that in the adult bone marrow (ABM), MPL is required by progenitor/stem cells for commitment and production of MKs, but not autonomously by the MK lineage for platelet production.
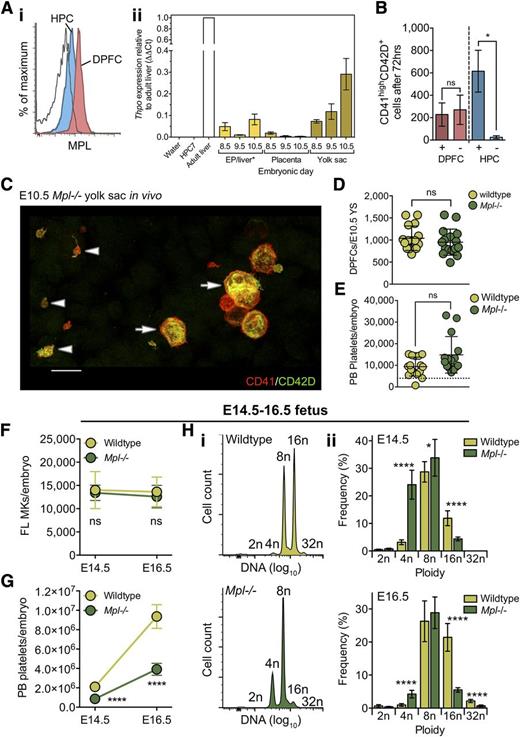
We investigated whether THPO-MPL signaling could influence YS DPFC and HPC lineages in vitro. Although expressed at a lower level than DPFCs, HPCs in the E10.5 YS were almost entirely MPL positive (Figure 6Ai) and by E10.5 Thpo was expressed in the YS at 30% of the adult level (Figure 6Aii); thus, local signaling is possible. Despite the relatively high expression of MPL by the DPFC population, we recently demonstrated that THPO is not required for in vitro proplatelet formation from DPFCs.28 To examine whether THPO was required for MK differentiation from HPCs in vitro, purified YS cells were cultured with and without THPO for 72 hours. Consistent with our previous findings, THPO-stimulated cells did not increase in DPFC numbers but were critical for MK formation from HPCs (Figure 6B).
MPL is dispensable for formation of DPFCs and MKs in vivo and for the first thrombopoietic phase, but is critical for the rapid expansion of platelet mass during late fetal life. (A) (i) Representative flow cytometry plot demonstrating the expression of MPL at high level by CD45−CD41highCD42D+ DPFCs (red) and at low level by CD45+CD41low HPCs (blue) in E10.5 YS cell suspensions. n = 3. Clear curve, isotype control for anti-MPL staining in the HPC population. (ii) qRT-PCR detection of Thpo expression in E8.5 to E10.5 tissues. Expression values are expressed using the ΔΔCt method relative to adult liver expression. Water and RNA from the HPC7 hematopoietic cell line were used as negative controls; RNA from adult liver was used as a positive control. n = 3. Bars, SEM. *RNA was extracted from whole embryo proper at E8.5 and E9.5; at E10.5, RNA was extracted from dissected livers. (B) Quantification of CD41highCD42D+ cells after culture of E10.5 YS DPFCs and HPC for 72 hours in vitro in the presence or absence of exogenous THPO (100 ng/mL). Data are cumulative of 3 independent experiments. ns, not significant. *P < .05. (C) Representative 3-dimensional projection of confocal z-stacks from a Mpl−/− E10.5 YS revealing the presence of both CD41highCD42D+ DPFCs (arrow) and platelets in vivo (arrow head). n = 15. Bar, 15 μm. (D-E) Quantification of (D) E10.5 YS DPFCs and (E) circulating E10.5 peripheral blood (PB) platelets in wild-type (n = 16) and Mpl−/− (n = 15) embryos. ns, not significant. Dashed line represents the upper limit of maternal platelet contamination to each sample. (F-G) Numbers of (F) CD41highCD42D+ MKs and (G) circulating peripheral blood platelets in wild-type and Mpl−/− embryos at E14.5 and E16.5 reveal that Mpl−/− fetuses are capable of generating normal numbers of MKs, yet exhibit an inability to generate normal numbers of circulating platelets. ns, not significant. ****P < .00001. n = 7 to 16 per genotype. (H) Representative histograms of CD41highCD42D+ liver MK DNA ploidy analysis from (i) E16.5 wild-type and Mpl−/− and (ii) quantitation of MK ploidy class frequencies at both E14.5 and E16.5 reveal that, although wild-type MKs reach up to 32n in vivo, Mpl−/− MKs exhibit a pronounced block at the 8n state.
MPL is dispensable for formation of DPFCs and MKs in vivo and for the first thrombopoietic phase, but is critical for the rapid expansion of platelet mass during late fetal life. (A) (i) Representative flow cytometry plot demonstrating the expression of MPL at high level by CD45−CD41highCD42D+ DPFCs (red) and at low level by CD45+CD41low HPCs (blue) in E10.5 YS cell suspensions. n = 3. Clear curve, isotype control for anti-MPL staining in the HPC population. (ii) qRT-PCR detection of Thpo expression in E8.5 to E10.5 tissues. Expression values are expressed using the ΔΔCt method relative to adult liver expression. Water and RNA from the HPC7 hematopoietic cell line were used as negative controls; RNA from adult liver was used as a positive control. n = 3. Bars, SEM. *RNA was extracted from whole embryo proper at E8.5 and E9.5; at E10.5, RNA was extracted from dissected livers. (B) Quantification of CD41highCD42D+ cells after culture of E10.5 YS DPFCs and HPC for 72 hours in vitro in the presence or absence of exogenous THPO (100 ng/mL). Data are cumulative of 3 independent experiments. ns, not significant. *P < .05. (C) Representative 3-dimensional projection of confocal z-stacks from a Mpl−/− E10.5 YS revealing the presence of both CD41highCD42D+ DPFCs (arrow) and platelets in vivo (arrow head). n = 15. Bar, 15 μm. (D-E) Quantification of (D) E10.5 YS DPFCs and (E) circulating E10.5 peripheral blood (PB) platelets in wild-type (n = 16) and Mpl−/− (n = 15) embryos. ns, not significant. Dashed line represents the upper limit of maternal platelet contamination to each sample. (F-G) Numbers of (F) CD41highCD42D+ MKs and (G) circulating peripheral blood platelets in wild-type and Mpl−/− embryos at E14.5 and E16.5 reveal that Mpl−/− fetuses are capable of generating normal numbers of MKs, yet exhibit an inability to generate normal numbers of circulating platelets. ns, not significant. ****P < .00001. n = 7 to 16 per genotype. (H) Representative histograms of CD41highCD42D+ liver MK DNA ploidy analysis from (i) E16.5 wild-type and Mpl−/− and (ii) quantitation of MK ploidy class frequencies at both E14.5 and E16.5 reveal that, although wild-type MKs reach up to 32n in vivo, Mpl−/− MKs exhibit a pronounced block at the 8n state.
We next asked whether MPL was required in vivo for production of either DPFCs or conventional MK lineages. To this end, we studied development in the constitutive Mpl−/− mouse line. We not only readily observed both DPFCs and platelets in whole-mount preparations of E10.5 YS (Figure 6C), but found that DPFCs were present at wild-type numbers (Figure 6D) and that E10.5 Mpl−/− embryos had normal numbers of circulating platelets (Figure 6E). This surprising finding demonstrated that, unlike in the ABM, MPL was dispensable for both DPFC formation from their primitive ancestors and for DPFC-derived thrombopoiesis in vivo.
Given that exogenous THPO is sufficient to induce megakaryopoiesis from YS HPCs in vitro, we next asked whether MPL was required by HPCs in vivo. To address this, we analyzed Mpl−/− fetuses at E14.5 and E16.5, time points that flank important landmarks in MK maturation and a spike in platelet formation (Figure 3E-G). We were again surprised to find that Mpl−/− liver MK numbers were completely normal (Figure 6F), but in contrast to the E10.5 embryo, numbers of circulating platelets were significantly lower at both E14.5 and E16.5 (Figure 6G). Thus, although THPO is sufficient to induce MK formation from HPCs in vitro, it is not required for this process in vivo.
Although Mpl−/− platelet numbers were lower compared with wild-type littermate controls, total numbers of circulating platelets did increase between E14.5 and E16.5. To gain a causative insight, we investigated how normal megakaryopoiesis had progressed in the absence of MPL. In contrast to wild-type MKs that achieved 16n and 32n genomes by E16.5, assessment of Mpl−/− MKs revealed a striking block of differentiation at the 8n stage (Figure 6Hi-ii). The inability of Mpl−/− MKs to mature to a higher ploidy state may explain the diminished capacity for platelet formation.
These experiments clarify the function of MPL in the prenatal organism and reveal that at no point is MPL required for the production of either of the platelet-forming lineages in vivo. Moreover, MPL is entirely dispensable for the first phase of DPFC-derived thrombopoiesis but is required during the fetal stages to successfully complete MK maturation and maximize platelet production.
Discussion
Limited knowledge of prenatal megakaryopoiesis and platelet formation has made the interpretation of the cellular etiology of neonatal thrombocytopenia problematic. We untook a comprehensive investigation into the spatial and temporal emergence of the mouse prenatal platelet-forming lineages and explored the developmental requirement for key molecular regulators of the adult megakaryocytic lineage. In particular, we elucidated the functions of THPO-MPL signaling during prenatal thrombopoiesis and identified a window of time in which MPL is essential for normal megakaryopoiesis.
In this study, we showed that CD41highCD42D+ DPFCs are present in all major hematopoietic sites of the embryo as early as E9.5. Because strong evidence exists to support a hypothesis that multiple sites of de novo hematopoiesis exist in the early embryo,44-46 we probed the potential of placenta, EP, and YS to autonomously generate CD41highCD42D+ cells in chemically defined medium. Our findings established that only the YS could independently produce large quantities of CD41highCD42D+ cells in vitro. Thus, between E8.5 and E9.5, DPFCs are produced in the YS and rapidly disseminate to other intra- and extraembryonic sites, thus founding the thrombopoietic environment seen by E10.5.
We and others have previously shown that the hallmark of multipotential HPC-derived megakaryopoiesis is the production of highly polyploid CD41highCD42D+ cells.27,28,47 In this study, we identified expression of CD42D by E10.5 YS CD45+CD41low HPCs as an effective differentiation marker: the CD45+CD41lowCD42D+ subpopulation exhibited diminished clonogenic potential and more rapid MK differentiation than their CD42D− counterparts. We found that the first highly polyploid MKs are seen as 8n cells in the E11.5 liver, which by E16.5 had achieved ploidy states up to 32n. This indicates that, although the YS environment was compatible with both DPFC and MK commitment, completion of HPC-derived megakaryopoiesis occurred many days later after seeding of the liver. Of note, given that HSCs are present in the E11.5 liver,59 when highly polyploid MKs first appear, it is possible that HSCs also contribute to MK population. Highly polyploid MK appearance coincided with a rapid increase in circulating platelets from E12.5 to E16.5, suggesting that HSPC-derived MKs supersede DPFCs as the dominant platelet producers from E12.5.
To address the question of whether DPFC and MKs, being distinct in ancestry, spatial location, and developmental time, were subject to distinct molecular regulatory controls, we compared the TF expression profile of each lineage. We found that, although many TFs were differentially expressed, a number of TFs that are considered critical to the adult MK lineage identity were similarly expressed by both populations. The finding that perturbed expression of Gata1 or Nfe2 within the DPFC lineage resulted in abnormal developmental progression, as previously observed in HSPC-derived MKs in the fetus and adult,18,34 established the principle that a group of core TFs exists that are critical to the development and function of all platelet-forming cells and that phenotypes that derive from these genetic lesions can manifest early in development, even before the initiation of HSPC-derived (definitive) hematopoiesis.
Recent controversy has arisen in the field regarding the role of THPO-MPL signaling in megakaryopoiesis and thrombopoiesis. Within the ABM MK lineage, constitutive deletions of THPO and MPL led to the idea that THPO-MPL was critical from HSPCs through to MKs and platelets.6-14 However, a recent study using in vivo Mpl deletion within either MKs or their progenitors showed that, although MPL is required for HSPC commitment to the MK lineage, it is dispensable in mature MKs.58 Surprisingly, we found that embryonic platelet forming lineages have very different in vivo requirements for THPO-MPL signaling. We found that not only was the primitive process of DPFC formation and the first wave of thrombopoiesis at E10.5 completely MPL independent, but HPC differentiation toward the MK lineage in the fetal liver was also MPL independent. We did, however, find that completion of megakaryopoiesis in the fetal liver was highly MPL dependent as Mpl−/− MKs did not achieve the highest ploidy states, resulting in a significantly diminished capacity to expand the circulating platelet mass. Whether this deficiency is MK autonomous or secondary to progenitor cell defects, as occurs in the adult,58 remains to be clarified.
The finding that MPL is dispensable for prenatal platelet-forming lineage production and early thrombopoiesis, but progressively becomes essential for optimal prenatal platelet mass and then during bone marrow hematopoiesis for MK formation58 provides potentially useful insight into why CAMT is a progressive disorder in early life. It will be of great future interest to ascertain whether the acquisition of MPL sensitivity observed in mouse prenatal platelet-forming cells also occurs in the pre/perinatal human.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Hanna Mikkola for sharing unpublished data, Drs Julie Sheridan and Christine Biben for critical comments on the manuscript, and Marion Lebois and Casey Ah-Cann for technical assistance. This work was supported by the Australian Research Council (ARC) Stem Cells Australia program, an ARC Discovery Early Career Research Award Fellowship (to S.T.), an ARC Strategic Research Australian Postgraduate Award studentship (to K.S.P.), and by a program grant (1016647), fellowships (to W.S.A. and D.J.H.), and Independent Research Institutes Infrastructure Support Scheme grant 361646 from the Australian National Health and Medical Research Council, the Australian Cancer Research Fund, and Victorian State Government Operational Infrastructure Support.
Authorship
Contribution: K.S.P., T.J.S., C.A.D., E.C.J., W.S.A., D.J.H., and S.T. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samir Taoudi, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne, VIC 3052, Australia; e-mail: taoudi@wehi.edu.au.