Key Points
CyBorD achieves excellent outcome in noncardiac patients with AL amyloidosis and can rescue subjects with reversible heart damage.
The outcome of high-risk patients remains poor, but response to CyBorD can also improve survival in this group.
Abstract
The combination of cyclophosphamide/bortezomib/dexamethasone (CyBorD) showed early promise of high rates of hematologic responses tempered by studies showing the inability to overcome poor prognosis of advanced cardiac amyloidosis. Large studies are needed to clarify its role in light chain (AL) amyloidosis. We report the outcome of 230 patients treated frontline with CyBorD. Overall hematologic response rate was 60%, and in the 201 patients with measurable disease it was 62%, with 43% achieving at least very good partial response (VGPR). Cardiac response was reached in 17% of patients and renal response in 25% of patients. Advanced cardiac stage III patients (amino-terminal pro-natriuretic peptide type B >8500 ng/L) had lower response rates (42%, ≥ VGPR 23%) and poorer survival (median, 7 months). Nevertheless, hematologic response improved survival in these subjects (67% at 2 years), showing the importance of striving for a good response even in this group.
Introduction
In amyloid light chain (AL) amyloidosis, clonal plasma cells use proteasomes to cope with misfolded light chain-induced proteotoxicity, and the proteasome inhibitor bortezomib is a potential targeted therapy.1,2 Early reports supported this expectation, showing high response rates, particularly upfront.3 A prospective trial showed that single-agent bortezomib is highly and rapidly effective.4 Combinations with alkylators are even more promising. Two studies, including 30 patients receiving cyclophosphamide/bortezomib/dexamethasone (CyBorD) frontline, reported hematologic response in 90% of cases, with ∼65% of patients reaching complete response (CR).5,6 This led to CyBorD becoming one of the regimens most commonly prescribed in AL amyloidosis. However, a subsequent study showed that in patients with advanced cardiac involvement, CRs are rarer and survival remains poor.7 Additionally, 2 parallel matched case-control studies comparing CyBorD and bortezomib/melphalan/dexamethasone (BMDex) with standards of care cyclophosphamide/thalidomide/dexamethasone (CTD) and melphalan/dexamethasone (MDex), showed that the higher rates of good quality hematologic response obtained with bortezomib-based regimens did not improve overall survival.8,9 Thus, there is the need for large studies to identify patients who benefit most from these combinations. We report the outcome of 230 newly diagnosed patients treated with CyBorD at the National Amyloidosis Centre (London, United Kingdom) and the Amyloidosis Research and Treatment Center (Pavia, Italy).
Study design
Our prospectively maintained databases were searched for newly diagnosed patients with AL amyloidosis treated with CyBorD. All 230 patients receiving this regimen between August 2006 and March 2013 were included. At the National Amyloidosis Centre, CyBorD substituted CTD as standard frontline therapy. In Italy, all patients not enrolled in clinical trials receive CyBorD or BMDex; cyclophosphamide is preferred in patients younger than 65 years with potentially reversible contraindications to autologous stem cell transplantation.10 Patients had biopsy-proven amyloidosis. The deposits were characterized as AL-type by immunohistochemistry, immunoelectron microscopy,11 or proteomics,12 and hereditary forms were excluded by DNA analysis.13 Patients gave written informed consent for use of their clinical data as approved by Ethics Committees.
The Mayo Cardiac Staging System was used for stratification.14 Furthermore, stage III patients were divided into 2 groups based on whether amino-terminal pronatriuretic peptide type-B (NT-proBNP) was below (stage IIIa) or above (stage IIIb) 8500 ng/L, which indicates very poor prognosis.15 Hematologic and cardiac response were assessed according to the International Society of Amyloidosis criteria.16 Measurable disease was defined as the difference between involved and uninvolved free light chain >50 mg/L for hematologic response and NT-proBNP >650 ng/L for cardiac response. Two patients with cardiac involvement and NT-proBNP <650 ng/L were not included in the calculation of cardiac response. Renal response was evaluated according to recent criteria.17 Treatment was discontinued in case of unsatisfactory response or toxicity. Statistical methods are reported in the supplemental Methods on the Blood Web site.
Results and discussion
Patients’ characteristics and treatment schedules are reported in supplemental Table 1. Briefly, 73% of patients had cardiac involvement (stage IIIa, 29%; stage IIIb, 20%), and 68% had renal involvement (stage III, 8%). Cyclophosphamide was administered orally at 300 mg/m2 weekly in all patients. Sixteen percent of patients received bortezomib 1.6 mg/m2 weekly or 1.3 mg/m2 twice weekly, the maximum tolerated doses in the prospective trial of bortezomib in AL amyloidosis,18 and 42% received 1.3 mg/m2 weekly or 1.0 mg/m2 twice weekly. Most patients (184 or 80%) received at least 80 mg of dexamethasone per week.
Twenty-three patients experienced severe adverse events (detailed in “Treatment toxicity” in the supplemental Data), and 29 patients (11, stage IIIa; 18, stage IIIb) died within 3 months of diagnosis.
By intent-to-treat, hematologic response was achieved in 138 of 230 patients (60%), with CR in 54 cases (23%). Among 201 patients with measurable disease, 125 (62%) responded (CR in 42, 21%; very good partial response [VGPR] in 45, 22%). Response rates were lower in stage IIIb patients (Table 1). In a landmark analysis excluding patients dying within 3 months, 126 of 174 patients (72%) responded, with 42 CRs (24%) and 45 VGPRs (26%). By intent-to-treat, after completing a median of 4 cycles, 29 patients (17%) achieved cardiac response and 40 patients (25%) achieved renal response (response by stage is reported in “Cardiac and renal response by stage” in the supplemental Data). Eight patients (32%) had liver response.
Hematologic response rate by intent-to-treat according to cardiac stage and bortezomib and dexamethasone dosages in 201 patients with measurable disease
| Response category . | Stage I (30 patients) . | Stage II (67 patients) . | Stage IIIa (61 patients) . | Stage IIIb (43 patients) . |
|---|---|---|---|---|
| Overall response | 23 (77%) | 43 (64%) | 42 (69%) | 18 (42%)* |
| CR | 10 (33%) | 12 (18%) | 14 (23%) | 6 (14%) |
| VGPR | 7 (23%) | 18 (27%) | 16 (26%) | 4 (9%) |
| PR | 6 (20%) | 13 (19%) | 12 (20%) | 8 (19%) |
| Response category . | Stage I (30 patients) . | Stage II (67 patients) . | Stage IIIa (61 patients) . | Stage IIIb (43 patients) . |
|---|---|---|---|---|
| Overall response | 23 (77%) | 43 (64%) | 42 (69%) | 18 (42%)* |
| CR | 10 (33%) | 12 (18%) | 14 (23%) | 6 (14%) |
| VGPR | 7 (23%) | 18 (27%) | 16 (26%) | 4 (9%) |
| PR | 6 (20%) | 13 (19%) | 12 (20%) | 8 (19%) |
| Response category . | Full bortezomib dose (35 patients) . | Intermediate bortezomib dose (82 patients) . | Low bortezomib dose (79 patients) . | |
|---|---|---|---|---|
| Overall response | 29 (83%) | 57 (69%) | 42 (53%)† | |
| CR | 12 (34%) | 20 (24%) | 11 (14%)† | |
| VGPR | 7 (20%) | 21 (26%) | 17 (21%) | |
| PR | 10 (29%) | 16 (19%) | 14 (18%) | |
| Response category . | Full bortezomib dose (35 patients) . | Intermediate bortezomib dose (82 patients) . | Low bortezomib dose (79 patients) . | |
|---|---|---|---|---|
| Overall response | 29 (83%) | 57 (69%) | 42 (53%)† | |
| CR | 12 (34%) | 20 (24%) | 11 (14%)† | |
| VGPR | 7 (20%) | 21 (26%) | 17 (21%) | |
| PR | 10 (29%) | 16 (19%) | 14 (18%) | |
| Response category . | Full dexamethasone dose (58 patients) . | Intermediate dexamethasone dose (102 patients) . | Low dexamethasone dose (41 patients) . | |
|---|---|---|---|---|
| Overall response | 45 (78%) | 62 (61%) | 20 (49%)† | |
| CR | 15 (26%) | 21 (21%) | 6 (15%)† | |
| VGPR | 17 (29%) | 23 (22%) | 5 (12%)† | |
| PR | 12 (21%) | 18 (18%) | 9 (22%) | |
| Response category . | Full dexamethasone dose (58 patients) . | Intermediate dexamethasone dose (102 patients) . | Low dexamethasone dose (41 patients) . | |
|---|---|---|---|---|
| Overall response | 45 (78%) | 62 (61%) | 20 (49%)† | |
| CR | 15 (26%) | 21 (21%) | 6 (15%)† | |
| VGPR | 17 (29%) | 23 (22%) | 5 (12%)† | |
| PR | 12 (21%) | 18 (18%) | 9 (22%) | |
Measurable disease is defined as a baseline difference between involved (amyloidogenic) and uninvolved free light chains >50 mg/L. Forty subjects with measurable disease died before evaluation of response and are considered nonresponders in the present intent-to-treat analysis. Data on bortezomib dosage were available in 196 of 201 patients with measurable disease. Cardiac staging is based on NT-proBNP (cutoff, 332 ng/L) and cTnT (cutoff, 0.035 ng/mL), or cTnI (cutoff, 0.1 ng/mL), with stage I, II, and III patients having none, one, or two markers above the cutoff, respectively. Stage IIIa patients have NT-proBNP ≤8500 ng/L and stage IIIb patients have NT-proBNP >8500 ng/L. Bortezomib dosage: full dose, 1.3 mg/m2 twice weekly or 1.6 mg/m2 once weekly; intermediate dose, 1.0 mg/m2 twice weekly or 1.3 mg/m2 once weekly; low dose, <1.0 mg/m2 twice weekly or 1.3 mg/m2 once weekly. Dexamethasone dosage: full dose, at least 160 mg per cycle; intermediate dose, <160 mg and ≥80 mg per cycle; low dose, <80 mg per cycle.
PR, partial response.
P < .05 compared with stages I, II, and IIIa.
P < .05 compared with full dose. Among the 15 patients who received 1.0 mg/m2 twice weekly, 2 achieved VGPR and 4 reached PR. Notably, in a multiple logistic regression analysis, only cardiac stage IIIb (P = .008) and not low doses of bortezomib (P = .191) and dexamethasone (P = .353), was an independent predictor of hematologic response, indicating that lower response rates in patients receiving lower doses of bortezomib or dexamethasone were likely explained by early deaths. Response rate was not affected by twice or once weekly administration (67% vs 62%; P = .549), or by IV or subcutaneous bortezomib administration (63% vs 61%; P = .862).
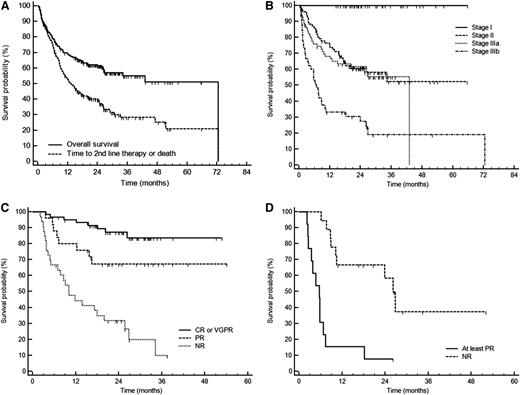
Overall, 55% of patients were projected to survive 5 years, and median time to second-line therapy or death was 13 months (Figure 1A). Cardiac stage determined the outcome (Figure 1B), and hematologic response significantly improved survival (Figure 1C-D). At multivariate analysis, stage IIIb was the only independent prognostic factor at baseline, whereas in the 3-month landmark analysis, hematologic response also independently predicted survival (supplemental Table 2).
Survival of 230 patients with AL amyloidosis treated with CyBorD. After a median follow-up of living patients for 25 months, 94 patients (41%) died. (A) Overall survival (cumulative proportion survival at 3 years, 55%) and time to second-line therapy or death (median, 13 months). (B) Survival according to cardiac stage. Cardiac staging is based on NT-proBNP (cutoff, 332 ng/L) and cTnT (cutoff, 0.035 ng/mL), or cTnI (cutoff, 0.1 ng/mL), with stage I, II, and III patients having none, one, or two markers above the cutoff, respectively. Stage IIIa patients have NT-proBNP ≤8500 ng/L and stage IIIb patients have NT-proBNP >8500 ng/L. Stage I, II, IIIa, and IIIb patients were 41, 77, 67, and 45, respectively. There were no deaths among stage I patients. Cumulative proportion survival at 3 years was 52% in stage II, 55% in stage IIIa, and 19% in stage IIIb. Survival of stage II and stage IIIa subjects was significantly shorter than that of stage I patients (P < .001). There was no difference in survival of stage II and stage IIIa patients (P = .613). The median survival of stage IIIb patients was 7 months (P < .001 compared with stages I, II, and IIIa). (C) Survival of 118 cardiac stage II and IIIa patients according to hematologic response (3-month landmark). Cumulative proportion survival at 3 years was 84% in patients reaching at least VGPR, 67% in subjects attaining PR (P = .042 compared with ≥ VGPR), and 10% in nonresponders (P < .001 compared with PR). The median survival of nonresponders was 10 months. (D) Survival of 31 cardiac stage IIIb patients according to hematologic response (3-month landmark). Median survival was 26 months for responders and 6 months for nonresponders (P < .001). The small number of patients did not allow discrimination between response categories. NR, no response; PR, partial response.
Survival of 230 patients with AL amyloidosis treated with CyBorD. After a median follow-up of living patients for 25 months, 94 patients (41%) died. (A) Overall survival (cumulative proportion survival at 3 years, 55%) and time to second-line therapy or death (median, 13 months). (B) Survival according to cardiac stage. Cardiac staging is based on NT-proBNP (cutoff, 332 ng/L) and cTnT (cutoff, 0.035 ng/mL), or cTnI (cutoff, 0.1 ng/mL), with stage I, II, and III patients having none, one, or two markers above the cutoff, respectively. Stage IIIa patients have NT-proBNP ≤8500 ng/L and stage IIIb patients have NT-proBNP >8500 ng/L. Stage I, II, IIIa, and IIIb patients were 41, 77, 67, and 45, respectively. There were no deaths among stage I patients. Cumulative proportion survival at 3 years was 52% in stage II, 55% in stage IIIa, and 19% in stage IIIb. Survival of stage II and stage IIIa subjects was significantly shorter than that of stage I patients (P < .001). There was no difference in survival of stage II and stage IIIa patients (P = .613). The median survival of stage IIIb patients was 7 months (P < .001 compared with stages I, II, and IIIa). (C) Survival of 118 cardiac stage II and IIIa patients according to hematologic response (3-month landmark). Cumulative proportion survival at 3 years was 84% in patients reaching at least VGPR, 67% in subjects attaining PR (P = .042 compared with ≥ VGPR), and 10% in nonresponders (P < .001 compared with PR). The median survival of nonresponders was 10 months. (D) Survival of 31 cardiac stage IIIb patients according to hematologic response (3-month landmark). Median survival was 26 months for responders and 6 months for nonresponders (P < .001). The small number of patients did not allow discrimination between response categories. NR, no response; PR, partial response.
Twenty patients received second-line therapy with lenalidomide/dexamethasone: 14 (70%) responded, including 3 refractory to CyBorD, with 2 CRs and 5 VGPRs. Seventeen patients, 7 of whom were refractory to CyBorD, underwent autologous stem cell transplantation: 11 (65%) responded, with 8 CRs (47%) and 1 (6%) VGPR. Four of 9 patients with cardiac involvement achieved cardiac response before transplant. There was no transplant-related mortality. Eleven relapsing patients received second-line bortezomib-based treatment, and in 3 patients VGPR was restored. An additional 8 patients had an immunomodulatory drug (thalidomide in 6 subjects and lenalidomide in 2) added to bortezomib/dexamethasone, and 7 responded (including 2 CRs and 3 VGPRs).
With 230 patients enrolled, the current report is the largest study of CyBorD in AL amyloidosis. As per most studies in this rare disease, our report is limited by retrospective design. In this “real-word” study of unselected subjects, overall response rates, particularly organ response, were lower than previously reported, and comparable to that observed with other regimens, such as MDex,8,19 CTD,9,20 and BMDex in the interim analysis of the randomized trial vs MDex.21 This was due to the inability of CyBorD to reduce early mortality in high-risk (stage IIIb) patients. However, CyBorD proved highly effective in patients without advanced cardiac involvement. We did not observe differences in survival between stage II and stage IIIa subjects, identifying an “intermediate-risk” group comprising the “old” stage II and stage IIIa patients and indicating the impact of treatment regimens in redefining staging systems. Importantly, CyBorD improved survival even in stage IIIb subjects who were able to receive enough treatment (ie, surviving at least 3 months from diagnosis), supporting the case for hematologic response to extend survival by preventing further worsening of cardiac damage. Timing of cardiac responses in stage IIIb remains unclear (low in our series at an early assessment time point) and may well be delayed in this advanced setting. This emphasizes the importance of striving for a good and rapid response even in this poor-risk group. In the present study, CyBorD appeared to be well tolerated. However, despite rigorous prospectively maintained databases, due to the retrospective nature of this study, safety data need to be interpreted with caution to avoid a serious risk of underestimating toxicity. Indeed, in the prospective ALChemy series, grades 3 to 4 toxicity with CyBorD occurs in ∼50% of patients.22 The present study also allowed some observations on second-line therapy after CyBorD. Cardiac response to CyBorD may allow second-line transplant in previously ineligible patients, and immunomodulatory drugs, particularly lenalidomide, combined with dexamethasone alone or added to bortezomib, appear to be good rescue agents in refractory subjects, in agreement with previous observations.23 In conclusion, for patients who cannot be enrolled into clinical trials, CyBorD is a useful, highly effective upfront option. Results of an ongoing trial comparing BMDex and MDex are eagerly awaited to prospectively confirm the utility of bortezomib/alkylators/steroid combinations in AL amyloidosis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the staff in histopathology, immunology, echocardiography, and genetics laboratories for their help with confirmation of diagnosis, as well as clinical fellows, nurses, and other staff for looking after the patients. The authors specially thank the hematologists who treated the patients reported in this study.
This study was supported in part by grants from the Associazione Italiana per la Ricerca sul Cancro, Special Program Molecular Clinical Oncology 5 per mille n. 9965 and CARIPLO “Structure-function relation of amyloid: understanding the molecular bases of protein misfolding diseases to design new treatments n. 2013-0964.”
Authorship
Contribution: G.P., G.M., and A.D.W. designed the study, evaluated patients, collected data, analyzed data, wrote the manuscript, and gave final approval; S.S., P.M., J.G., A.F., H.L., and M.B. evaluated patients, collected data, analyzed data, critically reviewed the manuscript, and gave final approval; and P.H. critically reviewed the manuscript and gave final approval.
Conflict-of-interest disclosure: G.M. received honoraria from Millennium-Takeda. G.P. and A.D.W. received honoraria from Janssen-Cilag. The remaining authors declare no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, Viale Golgi, 19-27100 Pavia, Italy; e-mail: gmerlini@unipv.it.