Abstract
Immune dysregulation is a cardinal feature of chronic lymphocytic leukemia (CLL) from its early stage and worsens during clinical observation, even in absence of disease progression. Although the mechanisms remain unclear, new insights are emerging into the complex relationship between the CLL clone and its immune environment. T cells are increased in early-stage disease and show progressive accumulation and exhaustion. The mechanisms that drive this expansion may include auto-antigens involved in the original clonal expansion. In addition, chronic viral infections such as cytomegalovirus generate huge virus-specific immune responses, which are further expanded in CLL. Attention is now focused largely on the direct immunosuppressive properties of the tumor. Remarkably, CLL clones often have features of the recently described regulatory B cells producing immunosuppressive IL-10. Better knowledge of the regulatory properties intrinsic to CLL cells may soon become more important with the switch from chemotherapy-based treatments, which trade control of CLL with further impairment of immune function, to the new agents targeting CLL B-cell receptor–associated signaling. Treatment with these new agents is associated with evidence of immune recovery and reduced infectious complications. As such, they offer the prospect of immunologic rehabilitation and a platform from which to ultimately replace chemotherapy.
Introduction
Chronic lymphocytic leukemia (CLL) is a tumor of mature B cells and comprises 2 major subsets with either unmutated (U-CLL) or mutated (M-CLL) immunoglobulin variable-region genes, indicative of an origin from pregerminal center (GC) or post-GC CD5+ B cells, respectively.1,2 Clinical progression differs between the 2 subsets, with U-CLL associated with a worse prognosis.3 Heterogeneity of clinical outcome is accompanied by a variable grade of B-cell receptor (BCR) anergy, measured as low sIgM levels and signaling capacity, which is evident in all CLL but is particularly prominent in M-CLL.4 Tumor cells express the phenotype of “activated” B cells,5 resembling B cells undergoing chronic antigenic stimulation.6,7 This is revealed by presumptive antigen-mediated downmodulation of sIgM, which is reversible after culture in vitro and is reflected in vivo where levels of sIgM determine the strength of signaling.4,8
Both U-CLL and M-CLL are associated with significant perturbations of the immune system, of which immune suppression is the most typical feature, from the early stages.9,10 Infections (∼60%) are the leading cause of death in CLL, and the incidence of secondary malignancies is also increased.11-14 Chemotherapy will clearly contribute to immune suppression in most patients, but perturbations within immune function are present from the early stages of disease, and only ∼30% of patients will require treatment.10 Hematopoietic-specific autoimmune responses are also observed in CLL and autoimmune hemolytic anemia is the most common, whereas nonhematologic autoimmune phenomena are extremely rare.15 Although clinically overt autoimmune hemolytic anemia may be observed before treatments, these disorders are often precipitated by chemotherapy, suggesting that moderate immune suppression can depress immune regulatory control of autoimmune responses. Interestingly, more intensive combination chemotherapy is protective against autoimmune phenomena and may indicate a more profound disruption of immune effector function.15
This perspective addresses the nature and potential etiology of the immunomodulation observed in association with CLL, and discusses how new targeted treatments alternative to chemotherapy hold the potential to reverse these effects.
Epidemiology of infections in patients with CLL
Before the widespread application of automated blood counting, the predominant clinical presentation of patients with CLL was with recurrent and often severe infections by common pathogens.12 The most commonly documented infections in treatment-naïve patients are respiratory tract and urinary tract infections, predominantly secondary to bacteria (67%), viruses (25%), and fungi (7%).12,15 Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenza underlie most cases of respiratory tract infection, with Escherichia coli implicated commonly in urinary tract infection.15 Herpesviruses are common chronic infections, but are found at a comparable prevalence with that of the general population.16 HSV seropositivity is almost ubiquitous and the risk of viral reactivation increases during the course of disease. Varicella zoster virus reactivation is a particular challenge, but is infrequent at diagnosis and becomes most apparent after initiation of treatment.15
Features of the innate immune response in patients with CLL
Quantitative and qualitative defects are seen within the innate and adaptive immune response in almost all patients from the time of diagnosis.12,15 Reduced levels of several complement proteins are observed, with specific reduction of at least some of the C1-C4 components in almost 40% of patients. The associated impairment in the ability to coat bacterial pathogens with C3b may contribute to the risk of infection.17 Importantly, this impairment in complement activity may also limit the therapeutic action of antibodies, which rely on complement-dependent cytotoxicity.18
A range of qualitative deficiencies of neutrophil function has been described including impaired phagocytic killing of nonopsonized bacteria and a reduction in C5a-induced chemotaxis.19 Interestingly, the circulating monocyte count is increased by >60% in patients with CLL,20 but such cells carry a “nonclassical” CD14+CD16++ phenotype and have a gene expression profile associated with immunosuppressive properties.20 Deficiencies in β-glucuronidase, lysozyme, and myeloperoxidase have been reported,12 associated with a relative “refractory” state with impaired response to classic pathogen-induced inflammatory responses.20,21 Although refractory to stimuli from normal B cells,22 monocytes can be driven to an immunosuppressive or “nonclassical” M2 macrophage-like phenotype upon engagement with CLL-derived soluble stimuli.20,23,24 Natural killer (NK) cells are also increased in the circulation of CLL patients but appear to have several functional defects,25 including impaired cytotoxic activity, possibly primarily because of defective expression of the NKG2D coreceptor.26 Nevertheless, NK cells are considered important for mediating the antitumor activity of CD20-specific monoclonal antibodies.
Features of the adaptive immune response in patients with CLL
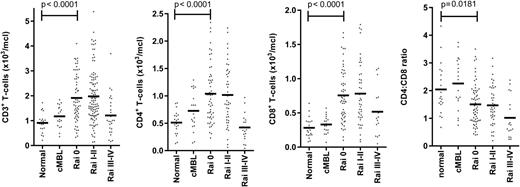
Marked dysfunction of the adaptive immune response is found in patients with CLL.12,15 Alterations within the T-cell repertoire are present at an early stage and become more apparent with disease progression. Interestingly, the absolute CD4+ and CD8+ T-cell counts are increased in early-stage disease, although the relatively higher increase in the CD8+ subset may lead to a reduction of the CD4+:CD8+ ratio (Figure 1).9,27,28 The mechanisms that underlie this increase in the peripheral T cell count are unknown but may relate to increased mobilization from secondary lymphoid tissue, or to an absolute increase in T cell numbers. Perhaps the most interesting explanation for the expansion of the adaptive immune response in the early phase of CLL would be that this represents a CLL-specific immune response. There has been a remarkable resurgence of interest in tumor-specific immune responses, particularly in disorders such as melanoma and lung cancer, and antibodies such as anti-PD-1 or CTLA-4, which overcome checkpoint blockade, are proving to be remarkably effective in managing these conditions. However, it has proven very difficult to demonstrate a CLL-specific immune response in vitro, and immunotherapy has not yet been proven in this condition. It is likely that the efficacy of checkpoint blockade will be assessed within CLL within the near future.
Early abnormalities of T-cell counts in patients with CLL. The histograms represent the paradoxical early expansions of CD3+, CD4+, and CD8+ T-cell subpopulations in the peripheral blood, and their CD4:CD8 ratio, in CLL patients at diagnosis. Each dot indicates an individual. Absolute values of CD3, CD4, CD8, and CD4:CD8 ratio are represented for cMBL (clinical monoclonal B-cell lymphocytosis), Rai stage 0, I-II, or III-IV CLL. Normal aged-matched donors (n = 57, age 52-83 y) on anticoagulation monitoring were used as internal reference values and for statistical comparison.
Early abnormalities of T-cell counts in patients with CLL. The histograms represent the paradoxical early expansions of CD3+, CD4+, and CD8+ T-cell subpopulations in the peripheral blood, and their CD4:CD8 ratio, in CLL patients at diagnosis. Each dot indicates an individual. Absolute values of CD3, CD4, CD8, and CD4:CD8 ratio are represented for cMBL (clinical monoclonal B-cell lymphocytosis), Rai stage 0, I-II, or III-IV CLL. Normal aged-matched donors (n = 57, age 52-83 y) on anticoagulation monitoring were used as internal reference values and for statistical comparison.
Chronic viral infections such as cytomegalovirus (CMV) trigger an increase in peripheral T cells in healthy donors, and may at least partly explain this feature. However, increased numbers of CD4+ T cells have been observed in the lymph nodes of CLL patients and can colocalize with CD38+ tumor cells, raising the possibility of local activation.29,30 Oligoclonal T-cell expansions are observed, but are also a feature in healthy elderly individuals and as such may simply represent a response to chronic infection.31
In addition to quantitative change, CD8+ and CD4+ T cells also show functional impairment in patients with CLL (reviewed by Riches and Gribben).14 In particular, there is markedly impaired helper activity.32 Differential gene expression is also observed compared with age-matched healthy donor T cells,33 particularly in genes involved in cytoskeletal formation and cytotoxicity pathways.33 This translates into impaired immunologic synapse formation with antigen-presenting cells as a result of defects in actin polymerization.34 However, more studies are required to investigate T-cell function within the CLL proliferation centers of the lymph nodes. The proportion of FoxP3+ regulatory T cells (Tregs) is increased in CLL, most notably in late-stage disease, and is likely to contribute to immune suppression.28,35
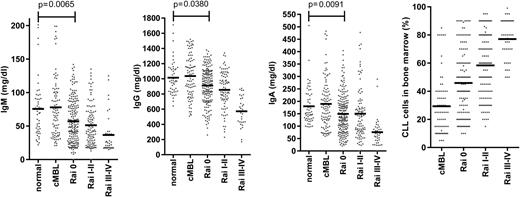
The major clinical parameter that correlates with infection risk in CLL is hypogammaglobulinemia.12 Levels of at least one serum immunoglobulin are diminished at an early stage of both CLL and small lymphocytic leukemia (SLL), despite initial low tumor load (Figures 2 and 3).9,36 The severity increases with the duration and progression of disease, and progresses to involve all Ig classes (IgG, IgA, and IgM), with a particularly marked fall in IgG3 and IgG4 subclasses.9,36,37 A direct correlation has been observed between low levels of IgG and IgA and increased morbidity and mortality from common bacterial infections, whereas the reduction in serum IgM levels seen early in disease does not seem to influence infection risk.36 However, trials of prophylactic intravenous immunoglobulins for CLL patients with hypogammaglobulinemia have not proven of major benefit,38 and one factor may relate to the low levels of IgA within the current therapeutic products.
Early reduction of serum immunoglobulin levels in patients with CLL. Serum IgM, IgG, and IgA levels in cMBL (clinical monoclonal B-cell lymphocytosis) and Rai stage 0, I-II, III-IV CLL at diagnosis are represented as dot plots. The normal aged-matched donor cohort was used as internal reference values and for statistical comparison. The bone marrow infiltration by CLL cells at the early and late stage of disease according to Rai classification is also represented.
Early reduction of serum immunoglobulin levels in patients with CLL. Serum IgM, IgG, and IgA levels in cMBL (clinical monoclonal B-cell lymphocytosis) and Rai stage 0, I-II, III-IV CLL at diagnosis are represented as dot plots. The normal aged-matched donor cohort was used as internal reference values and for statistical comparison. The bone marrow infiltration by CLL cells at the early and late stage of disease according to Rai classification is also represented.
Serum immunoglobulin levels in patients with SLL. Serum IgM, IgG, and IgA levels in normal individuals and in SLL patients at diagnosis.
Serum immunoglobulin levels in patients with SLL. Serum IgM, IgG, and IgA levels in normal individuals and in SLL patients at diagnosis.
Although the degree of hypogammaglobulinemia does not appear to differ between the U-CLL and M-CLL subsets,15,39 retrospective studies have documented an association between infection risk and immunoglobulin heavy chain variable (IGHV) gene status.13,40 In a study of 231 patients, those with U-CLL had increased infectious morbidity and mortality and shorter time to first infection compared with those with M-CLL.40 Another study of untreated patients showed that U-CLL tumors were correlated with shorter time to onset of recurrent infection.13 However, U-CLL progress more rapidly,3 and it remains difficult to exclude that the differences depend on different tumor load in the subsets.
Antibody responses to primary and secondary challenge with antigen are often inefficient from the early stages in CLL.41,42 Vaccination is most successful if immunoglobulin levels are preserved, and if conjugated vaccines are used.43 Notably, administration of live vaccines is potentially dangerous in patients with CLL.41 Unfortunately, modern conjugated vaccine preparations, which recruit T-cell “help” are still not generally capable of fully overcoming suboptimal immune responses.44 In a recent study, an adequate response to an initial dose of 13-valent pneumococcal conjugate vaccine was found in only 58% of CLL patients compared with all healthy age-matched controls.44 This study also observed a very poor increment of plasmablasts within peripheral blood at 1 week after vaccination. Overall the clinical observations suggest that both a direct suppression of B-cell function and an impairment of cognate T-cell help contribute to the poor response to vaccines. Conjugate preparations help to overcome some of these limitations but are not capable of restoring normal function.
Mechanisms of immune perturbations: tumor cells, infection, or both?
The degree of tumor burden is a dominant factor in relation to preservation of immune function. The most notable example is in relation to progression of hypogammaglobulinemia (Figure 2) or efficiency of vaccine response. An obvious explanation might be anatomic replacement of immune cells in primary and secondary lymphoid tissues by tumor cells. However, impairment of immune function is observed even during early-stage disease (Figure 2), when the degree of tumor infiltration within the marrow is relatively minimal.9 Reduced antibody levels are also seen in SLL despite minimal involvement of the hemopoietic compartments (Figure 3). As such other factors must play a significant role.
There is strong evidence for the importance of infection as an etiological factor in the development of mature B-cell tumors. Examples include the role of HTLV-1 (human T-cell leukemia virus) in adult T-cell leukemia/lymphoma, Epstein-Barr virus in endemic Burkitt lymphoma, and Borrelia burgdorferi in nasal-type B-cell lymphoma.45 No such direct causative link has been established between a specific infective agent and CLL, although disease risk is increased in those with hepatitis C infection.46 Nonetheless, it is possible that viral or bacterial infections may lead to the emergence of pathogen-reactive B cells, which subsequently undergo transformation.6,47 Reactivity of CLL-derived Ig from a small subset of M-CLL using IGHV3-7 with fungal β-(1,6)-glucan has also been reported.48 Interestingly, viral infection and autoimmune conditions can also expand populations of regulatory CD5+ B cells (Bregs),49 with which CLL cells share many features and may be related (discussed in the next section).
Viral infection also has a profound influence on the magnitude of major lymphoid subsets within healthy individuals,50 and drives the accumulation of oligoclonal CD4+ and CD8+ T-cell expansions.51-53 Several lines of evidence now suggest that T cells in patients with CLL have been driven strongly through many cell cycles of replication. In particular, a range of inhibitory receptors, such as PD-1, Tim3, Lag-3, and CTLA-4, have been identified on human T-cell subsets and are induced after sustained T-cell engagement, possibly as a mechanism to control the magnitude and effector function of immune responses.54 The reduced effector function of T cells that express these molecules has often been taken as a representation of T-cell “exhaustion.”54 Increased levels of PD1, CD160, and CD244 expression are observed on both CD4+ and CD8+ T cells in patients with CLL, and there is now considerable interest in understanding the mechanisms that drive this degree of T-cell expansions. Chronic infection must certainly be considered as a potential mechanism, and in this regard, the herpesvirus family, notably CMV, has emerged as a candidate of interest. CMV infection leads to the development of a huge CMV-specific T-cell response that is particularly expanded in patients with CLL,28,55 almost certainly as a response to increased levels of viral load (Helen Parry, manuscript submitted June 2015). However, PD-1 expression is actually decreased on effector T cells from patients with CLL, and CMV-specific CD8+ T cells retain high levels of functional activity.56,57 Indeed, PD-1 expression is not found to be increased on CMV-specific CD8+ cells,58 and as such the role of endogenous viruses as a mechanism to drive T-cell exhaustion remains uncertain.
Recently, bacterial contamination has emerged as a potential important mediator of chronic T-cell exhaustion in the setting of immune deficiency. In this regard, it is noteworthy that monocytes from patients show features of endotoxin tolerance, with low cytokine production and impaired inflammatory response to pathogens,21 which may facilitate bacterial translocation across the gut, leading to secondary exhaustion and functional impairment of CD4+ T cells.59 Another clue may come from the diffuse pattern of hypogammaglobulinemia, whereas an oligoclonal pattern might be expected if this were the result of chronic viral infection.60,61 As such, the current data point mainly to CLL tumor cells as the drivers of both T-cell exhaustion and indeed the other features of immune suppression.
Role of CLL cells in mediating immune suppression: the tumor as a regulatory B cell
A clear observation is that, in untreated patients, the severity of immune suppression in CLL increases with time from diagnosis and that this does not necessarily associate with tumor progression. As such, the policy of “watch and wait” before treatment is also acceptance of allowing an immune suppression to develop. One interpretation of this correlation is that the CLL tumor may directly suppress the natural function of cells of the immune system. Indeed, several studies have documented that CLL cells have such effects on normal T cells and B cells by direct cell contact and release of tumor-derived soluble factors.14 Tumor cells inhibit effector arms of the innate and acquired immune system through a variety of surface molecules, some of which have been summarized in Table 1. The mechanisms by which these molecules act on T cells have been reviewed elsewhere.14 Putative consequences on T cells are reduced proliferative capacity, inhibition of Th1 differentiation, and a relative skewing toward a Th2 response of peripheral blood T cells, expansions of CD25+ Tregs,78 and impaired immune synapse formation.14
Regulatory molecules of CLL cells
| Characteristic . | Proposed mechanisms . | Reference . |
|---|---|---|
| IL-10 | Regulates inflammation, autoimmunity, adaptive and innate immune responses, regulates production of tumor necrosis factor-α by macrophages | 24 |
| CD5 | Promotes STAT3/NFAT2-mediated IL-10 secretion | 62 |
| CD23 | Associates with regulatory B-cell phenotype | 24 |
| CD24 | Associates with regulatory B-cell phenotype | 24 |
| CD25 | Reduces T-cell proliferative capacity, inhibits Th1 differentiation, and skews peripheral T cells toward Th2 response in tissue by IL2 deprivation | 63 |
| CD27 | Enhances survival of Tregs; induces hypogammaglobulinemia by interacting with CD70 on plasma cells | 64,65 |
| CD39/CD73 | Ectoenzymes producing immune suppressive adenosine to inhibit T-cell proliferation | 66,67 |
| CD80/CD86 | Induced on activated CLL cells; induce T-cell tolerance | 14,68 |
| CD152 (CTLA-4) | Acts as an “off” switch signal via B7.1/B7.2 (CD80/CD86) to control T-cell responses | 69 |
| CD178 (FasL) | Induces hypogammaglobulinemia by CD95(Fas)-mediated suppression of primary plasma cell function | 70 |
| CD200 | Induces impaired actin synapse formation of T cells; inhibits IL2 and IFN-γ production by CD4+ T cells; promotes Treg differentiation of CD4+ T cells; inhibits antigen-presenting cells | 14,71 |
| CD269 (BCMA) | Favors plasma cell death and hypogammaglobulinemia by BAFF/APRIL deprivation | 72,73 |
| CD270 | Induces impaired actin synapse formation of T cells | 14 |
| CD274 (PD-L1) | Induces mpaired actin synapse formation of T cells; enhances susceptibility to apoptosis on CD4+ T cells at late stages of disease; induces hypogammaglobulinemia by plasma cell apoptosis | 14,74,75 |
| CD276 (B7-H3) | Induces impaired actin synapse formation of T cells | 14 |
| CD279 (PD-1) | Inhibits IFN-γ production by CD8+ T cells | 76 |
| NAMPT | Expressed on activated CLL cells, polarizes monocytes differentiation toward M2 macrophages | 23 |
| HLA-G | Associates with lower serum IgG, higher serum IL-10, and lower CD4+ T cells | 77 |
| Characteristic . | Proposed mechanisms . | Reference . |
|---|---|---|
| IL-10 | Regulates inflammation, autoimmunity, adaptive and innate immune responses, regulates production of tumor necrosis factor-α by macrophages | 24 |
| CD5 | Promotes STAT3/NFAT2-mediated IL-10 secretion | 62 |
| CD23 | Associates with regulatory B-cell phenotype | 24 |
| CD24 | Associates with regulatory B-cell phenotype | 24 |
| CD25 | Reduces T-cell proliferative capacity, inhibits Th1 differentiation, and skews peripheral T cells toward Th2 response in tissue by IL2 deprivation | 63 |
| CD27 | Enhances survival of Tregs; induces hypogammaglobulinemia by interacting with CD70 on plasma cells | 64,65 |
| CD39/CD73 | Ectoenzymes producing immune suppressive adenosine to inhibit T-cell proliferation | 66,67 |
| CD80/CD86 | Induced on activated CLL cells; induce T-cell tolerance | 14,68 |
| CD152 (CTLA-4) | Acts as an “off” switch signal via B7.1/B7.2 (CD80/CD86) to control T-cell responses | 69 |
| CD178 (FasL) | Induces hypogammaglobulinemia by CD95(Fas)-mediated suppression of primary plasma cell function | 70 |
| CD200 | Induces impaired actin synapse formation of T cells; inhibits IL2 and IFN-γ production by CD4+ T cells; promotes Treg differentiation of CD4+ T cells; inhibits antigen-presenting cells | 14,71 |
| CD269 (BCMA) | Favors plasma cell death and hypogammaglobulinemia by BAFF/APRIL deprivation | 72,73 |
| CD270 | Induces impaired actin synapse formation of T cells | 14 |
| CD274 (PD-L1) | Induces mpaired actin synapse formation of T cells; enhances susceptibility to apoptosis on CD4+ T cells at late stages of disease; induces hypogammaglobulinemia by plasma cell apoptosis | 14,74,75 |
| CD276 (B7-H3) | Induces impaired actin synapse formation of T cells | 14 |
| CD279 (PD-1) | Inhibits IFN-γ production by CD8+ T cells | 76 |
| NAMPT | Expressed on activated CLL cells, polarizes monocytes differentiation toward M2 macrophages | 23 |
| HLA-G | Associates with lower serum IgG, higher serum IL-10, and lower CD4+ T cells | 77 |
APRIL, a proliferation-inducing ligand; BCMA, B-cell maturation antigen; BAFF, B-cell activating factor; CTLA-4 cytotoxic T-lymphocyte–associated protein 4; IFN-γ, interferon-gamma NAMPT, nicotinamide phosphoribosyltransferase; NFAT2, nuclear factor of activated T-cells 2; PD-1, programmed cell death protein 1; STAT3, signal transducer and activator of transcription 3.
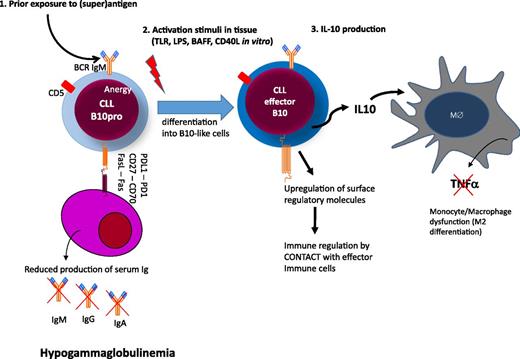
The mechanisms that underlie the development of hypogammaglobulinemia from the early stages of CLL are not clear. No single molecule or pathway has been implicated and this profile is likely to reflect the accumulation of a range of factors, which may suppress plasma cell number or function (Figure 4). CD27-CD70–mediated activation of the PI3K and MEK signaling pathway has been shown to reduce immunoglobulin secretion.64 CLL cells can also directly induce apoptosis of autologous plasma cells via CD95L(FasL)/CD95(Fas) interactions between CLL cells and plasma cells.70 We found evidence of both Fas-dependent and Fas-independent suppression of survival of human plasma cell lines when cocultured with CLL cells (Samantha Drennan and F.F., unpublished). However, adequate collection of autologous plasma cells from CLL patients for functional studies is difficult and makes this work technically challenging. A range of additional molecules such as CD25, CD44, CD69, CD95, or BCMA, which all have immunoregulatory potentials, are also upregulated on CLL cells and may contribute to the phenomenon.72 Interestingly, a subset of CLL cells also express PD-L1, which on Breg cells has been shown to regulate humoral immunity in an IL-10 independent fashion.74
Hypothetical model of immune regulation in CLL. Circulating CLL cells are anergic B cells with low surface IgM levels after exposure to (super)antigen. (1) CLL cells express several molecules that may have regulatory activity on plasma cells either by cell contact (eg, FasL, CD27, and/or or PDL1) or by deprivation of soluble factors essential for plasma cell survival (eg, BCMA, refer to text and Table 1).72,73 (2) Upon activation in tissue, CLL cells differentiate into B10 cells to produce immunosuppressive IL-10 and upregulate molecules with regulatory activity on other effector arms of the immune system. (3) Among the targets of IL-10, monocytes/macrophages are suppressed in their activity to produce tumor necrosis factor-α.
Hypothetical model of immune regulation in CLL. Circulating CLL cells are anergic B cells with low surface IgM levels after exposure to (super)antigen. (1) CLL cells express several molecules that may have regulatory activity on plasma cells either by cell contact (eg, FasL, CD27, and/or or PDL1) or by deprivation of soluble factors essential for plasma cell survival (eg, BCMA, refer to text and Table 1).72,73 (2) Upon activation in tissue, CLL cells differentiate into B10 cells to produce immunosuppressive IL-10 and upregulate molecules with regulatory activity on other effector arms of the immune system. (3) Among the targets of IL-10, monocytes/macrophages are suppressed in their activity to produce tumor necrosis factor-α.
An emerging theme regarding immunoregulation in CLL is the functional analogy between CLL and the recently described Breg cells. Breg cells form a small fraction of the normal B-cell repertoire and exert regulatory functions through soluble factors, predominantly IL-10.79 The fraction producing IL-10 has been termed B1080 and can represent as much as 4% of the B-cell pool.81 B10 cells differentiate from CD5+CD24hiCD27+ memory B cells, termed B10 progenitors (B10pro), which are induced to become IL-10-competent after stimulation through CD40 signaling. These cells expand significantly during inflammation,autoimmunity, and cancer to negatively regulate adaptive and innate immune responses.24 Although the presence of BCR engagement is not required to favor differentiation into effector B10 cells in vitro,79,80 adoptive transfer experiments have shown that prior antigen exposure is an essential element for the generation of Breg cells.82 Importantly, human B10 cells express low levels of sIgM,81 which is a consequence of the antigen engagement and a critical feature of B-cell anergy.83
CLL cells resemble Breg cells in many of these aspects. First, CLL cells are anergic B cells with low sIgM4,8 and demonstrate the basal functional features of B10 progenitors (B10pro cells) with variable capacity to act as effector B10 cells.24 In addition, the typical Breg markers of CD5, CD24, and CD27 are all defined within the typical phenotype of CLL (Table 1).24,62 Finally, although they spontaneously release modest amounts during circulation,84 CLL cells produce significant amounts of IL-10 after stimulation by a range of stimuli, including microbial products (eg, CpG-ODN, LPS), T-cell costimulation (via CD40L or IL4), or B cell–activating factor of the tumor necrosis factor family (BAFF) from monocyte/macrophages in vitro (Figure 4).24 Although these stimuli may not be operating in vivo, IL-10 transcript is upregulated within CLL cells of the lymph node,69 and STAT3, a potent mediator of IL-10-driven antiinflammatory signaling,85 is also phosphorylated in the tissues of CLL patients.86-88
The key determinants in vivo that drive the differentiation of CLL cells from a B10pro cell state into an effector B10 cell state are not yet clear.80 Mice models indicate that prior antigen exposure is required for proper differentiation into B10 cell,89 but it is likely that the strength of signaling through the BCR is another key factor. This differs between U-CLL and M-CLL because M-CLL tumors are generally more anergic than U-CLL tumors.4,8 However, the only study that has assessed IL-10 production from CLL cells in relation to IGHV sequence showed that M-CLL tumors generally produce more IL-10 than U-CLL.24 This observation merits further investigation and it appears possible that epigenetic programming is an additional important determinant that links B-cell signaling with IL-10 production (Samantha Drennan, Chris Oakes, and F.F., manuscript in preparation).90 Although T cell help is important in the differentiation of B10pro cells into effector B10 cells,89 CLL cells resemble anergic B cells operating in a T cell–independent fashion, suggesting that classical cognate T cell help is unlikely to contribute.6 One important factor may be BAFF, which is also expressed on monocytes/macrophages within proliferating centers and promotes B-cell activation, proliferation, and IL-10 production.91 Whatever its origin, IL-10 is a potent immunosuppressive molecule and it is perhaps not surprising that IL-10 levels have been associated directly with survival in patients with CLL.92
CLL cells also express other molecules associated with Breg activity including CD39 and CD73 ectoenzymes, CD200, CD25, CTLA4, and PD1, and these are often found at particularly high levels within tissue (Table 1).5,24,69,71 We find that expression of some molecules, such as PD1, increases during circulation before tissue entry, whereas others, including CD25 or CTLA4, increase after stimulation with CpG or BCR (unpublished). Similarly, the enzyme nicotinamide phosphoribosyltransferase (NAMPT) can be produced as an extracellular form by CLL lymphocytes upon BCR, Toll-like receptor, or nuclear factor κB activation, and polarizes monocyte differentiation toward M2 macrophages, further reducing T-cell responses and serving to sustain CLL survival by producing immunosuppressive IL-10.23
Improving the management of CLL: immunology comes center stage
The current backbone of CLL therapy relies on the use of chemotherapy agents, such as alkylating drugs or fludarabine, combined with anti-CD20 monoclonal antibodies. Although they have proven efficacy in symptomatic control of disease, these strategies do have a negative impact on immune function. As such, the consequences of these treatments on overall immune function in the patient with CLL will depend on the balance between the improvement that is gained from reduction in tumor burden and with the side effects of therapy. Overall there is a net accumulation of infectious episodes over time and a failure to reverse the underlying features of immune suppression.
Lenalidomide was perhaps the first example of how the immune system may be harnessed in the management of CLL. The drug has several immunomodulatory activities and its use is often associated with an acute inflammatory reaction. The features of this “tumor flare” include upregulation of CD40 and reduced expression of PD-L1 on the tumor cell, T-cell activation, and improvement in immune synapse formation and tumor lysis.
The subsequent introduction of novel targeted therapies with inhibitors of BCR-associated kinases now offers an opportunity for a reappraisal of the consequences of treatment on immune function. Phosphoinositide 3-kinase δ (PI3Kδ) can be targeted by the highly effective oral inhibitor idelalisib. PI3Kδ is expressed in several lymphoid subsets and its inhibition might therefore be expected to negatively affect immune competence. Instead, no overt increase in infection rate has been observed during treatment, and there may be some improvement in immune parameters. Despite these observations, immune dysregulation may be a concern and it is likely that the side effect of colitis reflects the consequence of impaired gut immunity and tolerance to commensal microbiota.
Another key enzyme is Bruton’s thyrosine kinase (BTK), which plays an essential role in BCR signaling in normal and CLL B cells, and which is targeted by ibrutinib. Therapeutic efficacy is obtained initially by the death of tumor cells within lymph node and bone marrow,93 although a significant proportion of tumor cells egress and persist as nondividing cells in the circulation up to several months during treatment.93,94 Current studies of patients taking ibrutinib suggest that there is recovery of humoral immunity, with an increase in serum immunoglobulin levels, predominantly of the IgA isotype, and a gradual significant decrease in infection rate, even when the circulating CLL count remains elevated.95,96 This may reflect the ability of bone marrow plasma cells to recover function at least partially, when relieved of suppressive activity of the CLL clone. However, BTK is important in immune tolerance and the potential development of autoimmune antibodies is something that should be monitored in future studies,97 particularly in light of the observation that ibrutinib may induce immunogenic cell death.98 In addition, it should be noted that congenital BTK deficiency leads to marked impairment of B-cell development, hypogammaglobulinemia, and recurrent infection,99 and the effect of chronic drug inhibition on the adult humoral immune system remains unclear.100 Ibrutinib also targets interleukin-2–inducible kinase within T cells, suggesting possible off-target effects that could contribute to disruption of immune homeostasis in the longer term.
Conclusions
Perturbation of the immune system is a dominant feature of CLL, but the complex mechanisms that mediate this are still largely unknown. Immune suppression is revealed by predisposition to infection. Profound alterations are observed within the immune repertoire of CLL patients and it will be important to determine to what extent this may reflect an attempt of the tumor cells to evade immune control. Importantly, CLL cells resemble natural Breg cells in many ways, and attention is now focusing on the direct immunosuppressive properties of the tumor compartment. Regulatory activity may be increased after local activation at proliferation centers within secondary lymphoid tissue, and future efforts should focus on the importance of tissue microenvironment in relation to local immune response. Finally, the new oral inhibitors of B-cell signaling appear to offer the potential for some recovery of endogenous immune function, although their use will require careful monitoring of the consequences of chronic suppression of natural B-cell function.
Acknowledgments
The authors thank Prof. Freda Stevenson for helping in the preparation and for critical review of the manuscript.
This study was supported by Leukaemia & Lymphoma Research, the Southampton Cancer Research UK, the NIHR Experimental Cancer Medicine Centres, Istituto Toscano Tumori (Florence, Italy), and the Hairy Cell Leukemia Research Foundation (United States).
Authorship
Contribution: F.F. and P.M. designed research, reviewed literature, shared data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Forconi, Cancer Sciences Unit, University of Southampton, Cancer Research UK Centre, Somers Cancer Research Building, MP824, Southampton General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: f.forconi@soton.ac.uk.