In this issue of Blood, Mammadova-Bach et al show that polymerized fibrin binds to the platelet receptor glycoprotein VI (GPVI), amplifying thrombin generation and enhancing thrombus growth.1
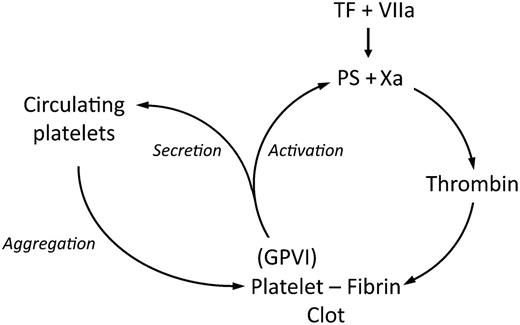
GPVI amplifies thrombin generation and platelet aggregation. PS, phosphatidylserine; TF, tissue factor.
GPVI amplifies thrombin generation and platelet aggregation. PS, phosphatidylserine; TF, tissue factor.
A large body of evidence shows that GPVI, a 58-kDa transmembrane protein in platelets, is the major receptor for collagen, and its interaction with type I and III fibrillar collagens leads to stable platelet adhesion and thrombus growth.2 Platelet adhesion is initiated at the site of vascular injury by the interaction of von Willebrand factor with the GPIb-V-IX complex on the platelet surface,3 which captures circulating platelets in high fluid shear to the site of injury. Transient platelet adhesion facilitates engagement of α2β1 and GPVI with exposed collagen in the wound site and these interactions lead to stable platelet adhesion. Interaction of GPVI with collagen activates the adherent platelets, triggers secretion, exposes procoagulant phospholipid on the platelet surface, and recruits more circulating platelets to the thrombus.
Despite these important functions of GPVI in initiating primary hemostasis, individuals with congenital GPVI deficiency only show very mild bleeding tendency,4 which is explained in part by the presence and compensatory function of other collagen receptors on platelets. Consistent with the mild phenotype observed in humans, GPVI knockout mice also show only slightly prolonged bleeding times but no overt bleeding.5 Surprisingly, GPVI-deficient mice are protected from experimental thrombosis induced by either mechanical arterial injury or exposure to ferric chloride6 or in a model of lethal thromboembolism.7 Furthermore, GPVI deficiency is also protective of acute thrombosis induced by atherosclerotic plaque rupture in ApoE-deficient mice.8 These protective effects are ascribed to reduced thrombus growth and thrombin generation. These unexpected results also question the role of collagen as an agonist for GPVI because there is no fibrillar collagen in a growing thrombus. If not fibrillar collagen, what is the physiologic agonist for GPVI in a growing thrombus? Despite this gap in knowledge, beneficial antithrombotic effects and low risk of bleeding suggest that inhibiting GPVI would be a promising approach in treating thrombosis. Thus, reagents that target the collagen binding site in GPVI and anti-GPVI antibodies have been developed and these reagents show efficacy in treating thrombosis in clinical studies. However, the long-sought-after physiologic agonist for GPVI has not been identified.
In this issue, Mammadova-Bach et al provide evidence that the physiologic ligand for GPVI in the platelet thrombus is polymerized fibrin. The GPVI-fibrin interaction promotes thrombus growth by 2 reciprocal amplification loops (see figure): (1) by exposing procoagulant phospholipid on the platelet surface, enhancing assembly of the tenase and prothrombinase complexes, promoting thrombin generation, and increasing fibrin deposition; and (2) by triggering platelet secretion and recruiting additional circulating platelets to the growing thrombus. Using a purified system of washed platelets and coagulation factors, these investigators show that thrombin generation is dependent on platelets and is significantly inhibited by GPVI blockade. Using normal and afibrinogenemic plasma, they show thrombin generation depends on fibrinogen, and is inhibited by the Gly-Pro-Arg-Pro peptide, a specific inhibitor of fibrin polymerization. Additional experiments confirm that GPVI binds only polymerized fibrin and that the antibody Fab 9O12 used in GPVI blockade also inhibits the GPVI-fibrin interaction. Using normal and GPVI-deficient platelets, and GPVI-blocking antibodies, these investigators further show that the GPVI-fibrin interaction leads to platelet spreading and stable activation, accompanied by phosphatidylserine exposure and recruitment of circulating platelets to the fibrin-platelet clot. The ability of GPVI to bind polymerized fibrin and not fibrinogen at high wall-shear rates distinguishes this interaction from that of integrin αIIbβ3 with fibrinogen. Simultaneous blockade of αIIbβ3 with abciximab and GPVI with Fab 9O12 reduces thrombin generation additively, suggesting that both of these receptors contribute to thrombus growth but by independent and complementary mechanisms. By identifying polymerized fibrin as the long-sought-after physiologic ligand for GPVI, these investigators establish a novel mechanism for the function of GPVI. These new insights show GPVI at the intersection between primary hemostasis and the coagulation cascade, and provide the basis for the design of safe and effective antithrombotic drugs.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal