In this issue of Blood, Krivega et al establish a new method to reactivate fetal hemoglobin (HbF) production in adult human erythroid cells through pharmacologic manipulation of chromatin looping at the β-globin locus.1
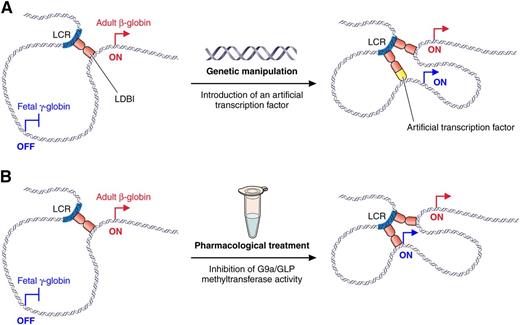
Different approaches to reactivate fetal γ-globin gene expression through alteration of chromatin looping. (A) Reactivation of fetal γ-globin transcription through the introduction of an artificial transcription factor that tethers the γ-globin promoter to the LCR as previously described.6 (B) Reactivation of fetal γ-globin transcription through pharmacologic inhibition of H3K9 methyltransferases as described in Krivega et al. Professional illustration by Patrick Lane, ScEYEnce Studios.
Different approaches to reactivate fetal γ-globin gene expression through alteration of chromatin looping. (A) Reactivation of fetal γ-globin transcription through the introduction of an artificial transcription factor that tethers the γ-globin promoter to the LCR as previously described.6 (B) Reactivation of fetal γ-globin transcription through pharmacologic inhibition of H3K9 methyltransferases as described in Krivega et al. Professional illustration by Patrick Lane, ScEYEnce Studios.
Sickle cell disease (SCD) and β-thalassemia are widespread genetic disorders that result from inherited mutations in the adult β-globin gene. An important aspect of SCD and β-thalassemia is that disease-causing mutations affect the adult β-globin gene but leave intact its fetal counterparts Gγ- and Aγ-globin, a point that explains why SCD and β-thalassemia patients first experience major symptoms in late infancy when the fetal γ-globin genes become developmentally extinguished.2 Furthermore, rare mutations that lead to persistence of fetal γ-globin expression in adults significantly ameliorate SCD and β-thalassemia symptoms, highlighting the clinical benefits of elevated levels of HbF.2 Therefore, a major research objective is the development of methods to reactivate fetal γ-globin in adult erythroid cells.
The β-like globin genes reside in a single cluster where they are arranged in the order of their expression during development. High-level expression of these genes is mediated by the locus control region (LCR), a distal array of multiple enhancers that act in an additive manner to increase the rate of transcriptional elongation.3 During development, when the embryonic, fetal, and adult β-globin genes undergo sequential phases of expression followed by gene silencing, the LCR alters its spatial positioning within the nucleus to remain in close proximity to the promoter of the developmentally appropriate, active β-like globin gene through a 3-dimensional looping of chromatin.4 Although the mechanism through which looping is established is not entirely clear, the authors have previously identified the Lim-domain binding 1 (LDB1) protein as a key factor that mediates loop formation.5 Furthermore, it has been shown that in adult erythroid cells, tethering the dimerization domain of LDB1 to the fetal γ-globin gene promoters via an artificial zinc-finger protein brings the LCR in close proximity to the fetal genes and stimulates their expression.6 Although this shows that forced looping through an artificial transcription factor allows reactivation of HbF in adult erythroid cells (see figure), such an approach requires genetic manipulation of erythroblasts, which may complicate its application in a clinical setting.
Here, Krivega et al describe a novel pharmacologic approach to modulate β-globin gene expression where they use a small molecule inhibitor of the histone H3 lysine 9 (H3K9) methyltransferase enzymes G9a and G9a-like protein (GLP) to reactivate HbF production in adult erythroid cells.1 Interestingly, the authors show that this reactivation is associated with spatial reconfiguration of the locus whereby the LCR alters its nuclear positioning to gain proximity to the fetal γ-globin genes (see figure). This finding is important because it provides proof-of-principle that structural reconfiguration of the β-globin locus can be achieved through pharmacologic modification of its chromatin state. In addition, the study provides new insights into the mechanism of long-distance enhancer-gene communication by showing that the chromatin-modifying enzyme G9a, previously shown to spread across the β-globin locus,7 contributes to the regulation of chromatin loop formation. This finding offers the first clue that chromatin spreading and looping may be functionally linked.
G9a and its paralog GLP are methyltransferases that can mono- and di-methylate H3K9. Furthermore, G9a and GLP possess ankyrin repeat domains, which allow them to bind to their own substrate, albeit with different specificities (ie, H3K9me1 for GLP and H3K9me2 for G9a). It has been previously shown that G9a is recruited to the β-globin LCR by the transcription factor NF-E2, and spreads across the β-globin locus.7 Furthermore, knocking down G9a through RNA interference in murine erythroid cells,7 or inhibiting its enzymatic activity in human hematopoietic progenitors,8 leads to reactivation of the embryonic/fetal β-like globin genes, suggesting that pharmacologic inhibition of G9a could be used to counteract fetal γ-globin silencing.
To determine the phase of erythropoiesis at which inhibition of G9a is most efficient to increase levels of HbF, the authors used a 3-stage ex vivo differentiation system with human CD34+ hematopoietic progenitors from adult donors. They demonstrate that inhibition of G9a/GLP methyltransferase activity with the small molecule inhibitor UNC06389 leads to a pronounced increase in HbF (up to 30% of total hemoglobin) when applied at the time of erythropoietin-mediated induction of erythroid differentiation. This effect is mediated through upregulation of fetal γ-globin and downregulation of adult β-globin expression. At the molecular level, the authors show that the drug leads to a locus-wide decrease in H3K9me2, which is accompanied by complex changes in G9a binding (ie, increased binding at the fetal promoter, decreased binding at the adult promoter, and no change at the LCR). Similarly, they observed a shift in binding of the looping factor LDB1 from the adult to the fetal gene promoters. Finally, they show that the fetal γ-globin gene relocates to achieve closer proximity to the LCR.1
Taken together, these results establish G9a as a major player in the maintenance of γ-globin silencing in adult erythroid cells. Furthermore, it suggests a mechanism whereby the G9a-mediated H3K9me2 mark on the γ-globin promoter prevents spatial proximity with the LCR through inhibiting binding of the “looping factor” LDB1. Testing this model will require additional experiments to determine the sequential order of events following H3K9 methyltransferase inhibition.
Finally, a surprising finding is that G9a remains bound to the β-globin locus upon drug treatment, despite a widespread loss of the H3K9me2 mark. This result is at odds with the current model of G9a spreading on chromatin through its ankyrin domain-mediated recognition of H3K9me2. However, it is consistent with the absence of a visible phenotype in knock-in mice carrying a mutant form of G9a that is unable to bind to H3K9me2.10 Although these results converge to suggest that the interaction of G9a with its substrate does not play a dominant role in the maintenance of G9a binding to chromatin in vivo, we cannot exclude the possibility that the interaction with H3K9me2 is important for the initial establishment of G9a binding (ie, spreading). A candidate factor for retaining G9a binding to the β-globin locus in the absence of H3K9me2 is G9a-heterodimerization partner GLP, whose H3K9me1 binding activity appears to play a dominant role in vivo.10 In that regard, it will be interesting to analyze GLP binding and H3K9me1 enrichment on the β-globin locus upon inhibition of H3K9 methyltransferase activity.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal