Key Points
RN-1 treatment of SCD mice results in increased human fetal γ-globin induction and fetal hemoglobin synthesis.
RN-1 treatment of SCD mice significantly reduces sickling, hemolysis, and tissue injury with no obvious adverse side effects.
Abstract
Inhibition of lysine-specific demethylase 1 (LSD1) has been shown to induce fetal hemoglobin (HbF) levels in cultured human erythroid cells in vitro. Here we report the in vivo effects of LSD1 inactivation by a selective and more potent inhibitor, RN-1, in a sickle cell disease (SCD) mouse model. Compared with untreated animals, RN-1 administration leads to induced HbF synthesis and to increased frequencies of HbF-positive cells and mature erythrocytes, as well as fewer reticulocytes and sickle cells, in the peripheral blood of treated SCD mice. In keeping with these observations, histologic analyses of the liver and spleen of treated SCD mice verified that they do not exhibit the necrotic lesions that are usually associated with SCD. These data indicate that RN-1 can effectively induce HbF levels in red blood cells and reduce disease pathology in SCD mice, and may therefore offer new therapeutic possibilities for treating SCD.
Introduction
Sickle cell disease (SCD) is the most common inherited human hematologic disorder, and is caused by a missense mutation in the adult β-globin gene that leads to altered biochemical characteristics of hemoglobin. Sickled erythrocytes are subject to premature destruction leading to hemolytic anemia, and can occlude blood flow, causing acute pain, disability, and chronic damage of various organs in SCD patients.1,2
Clinical studies have shown that increased synthesis of fetal hemoglobin (HbF) in sickled red blood cells (RBCs) leads to diminished severity of many clinical features of SCD.3,4 Therefore, therapeutic agents that can increase HbF production will be beneficial to SCD patients. Drugs such as hydroxyurea (HU),5-10 decitabine (DAC),11-14 and butyrates15-18 have been used for such purposes to treat SCD patients. HU is currently the only U.S. Food and Drug Administration (FDA)–approved HbF-inducing drug for individuals with SCD.19-22 However, the ability of HU to increase the number of HbF-containing reticulocytes is highly variable.23,24 Therefore, more consistently effective and improved HbF inducers are highly desired.
We previously reported that lysine-specific histone demethylase 1 (LSD1/KDM1A) and DNA methyltransferase 1 (DNMT1) physically interact with the nuclear receptor NR2C1 (TR2) and/or NR2C2 (TR4) to form a “core” tetrameric complex that recruits multiple additional corepressors to the ε- and γ-globin gene promoters and impart silencing and molecular repression to those genes in adult, definitive erythrocytes.25 LSD1 is a monoamine oxidase that contains an amine oxidase domain that catalyzes the flavin adenine dinucleotide (FAD)-dependent oxidation of amine substrates. It removes methyl groups from mono- and dimethyl histone H3 lysine 4 or 9 (H3K4 or H3K9, respectively), which are epigenetic markers that correlate most frequently with gene silencing.26,27
A monoamine oxidase inhibitor called tranylcypromine (TC), which is currently FDA-approved and prescribed for major depressive disorders, is a selective inhibitor of LSD1 with a half-maximal inhibitory concentration (IC50) of <2 µM.28 TC is known to cause very adverse clinical side effects when taken in conjunction with foods containing a high tyramine content (eg, yeast extract, red wine).29 Recently, we reported that inhibition of LSD1 by TC could enhance HbF synthesis in vitro in a dosage-dependent manner in primary human erythroid cells, as well as in mice bearing the human β-globin locus as a yeast-artificial-chromosome (YAC) transgene (β-YAC mice).30 However, high TC concentrations can lead to delayed erythroid maturation and a decline in total β-like globin mRNA in differentiating erythroid cell cultures.31 Although these in vivo data indicated that the inhibition of LSD1 could have clinical relevance for the treatment of SCD, it is also apparent that it is necessary to identify safer and more potent LSD1 inhibitors.
RN-1 is a cell-permeable TC analog that has been reported to act as a potent, irreversible inhibitor of LSD1 with a much lower IC50 (0.07 µM) than TC (2 μM).32 We therefore investigated the in vivo effects of RN-1 on γ-globin gene expression and erythroid physiology in a transgenic mouse model of SCD.33 These SCD mice express human α- and sickle βs-globin, and therefore mimic many of the genetic, hematologic, and pathophysiologic features that are found in human SCD patients, including irreversibly sickled RBCs, hemolytic anemia, high reticulocyte count, hepatosplenomegaly, and other organ pathology.33
Here we report that a robust increase of human fetal γ-globin and murine embryonic εy- and βh1-globin mRNAs and human HbF is observed in SCD mice after repeated RN-1 treatment. Furthermore, sickled RBCs and reticulocytes are significantly reduced, whereas the lifespan and the number of mature erythrocytes increased markedly in the peripheral blood of RN-1–treated SCD mice, leading to significantly diminished pathophysiologic characteristics (hemolysis, splenomegaly, and organ necrosis) that are usually pronounced in untreated SCD mice. In addition, and unlike the negative effects of high TC levels,30 we did not observe overt adverse side effects in SCD mice under the tested RN-1 drug regimen. These results provide an additional proof-of-concept with a possibly safer drug that modulating LSD1 activity, or the signaling pathways that it regulates, in SCD patients is a promising therapeutic approach to induce persistent fetal γ-globin accumulation and thus alleviate the associated disease pathophysiology.
Methods
Mice
SCD mice (6-8 weeks old) were purchased from the Jackson Laboratory (stock number: 013071) and used in all experiments. All animal experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Blood analysis
Blood was collected by retro-orbital bleeding, and hemoglobin composition, complete blood cell count, and reticulocyte count were measured as described.34,35 RBCs were labeled in vivo using N-hydroxysuccinimide biotin (Pierce),36-38 and the lifespan of circulating RBCs was measured as described in the supplemental Methods, available on the Blood Web site. Whole blood cells (10 μL) were stained with antibodies against mouse CD71 (BioLegend) and Ter119 (BioLegend), or human HbF (Invitrogen). Analytical flow cytometry was performed as described.35
QRT-PCR and ChIP analysis
Total RNA was prepared from 10 μL of whole blood. Synthesis of complementary DNA and quantitative real-time polymerase chain reaction (QRT-PCR) was performed as described.35 mRNA abundance of target genes was determined based on cycle threshold (CT) values of the QRT-PCR and then normalized to the abundance of Ornithine decarboxylase antizyme (Oaz1) mRNA as the internal control.39 Bone marrow (BM) cells were isolated from untreated and drug-treated SCD mice for chromatin immunoprecipitation (ChIP) assays as previously described.25 Antibodies (from Abcam) recognizing LSD1, dimethyl H3K4 (H3K4me2), dimethyl H3K9 (H3K9me2), histone H3 (H3), corepressor for element-1-silencing transcription factor (CoREST), DNMT1, TR2, and TR425 were purchased from Abcam for use in immunoprecipitation. Primers for QRT-PCR are shown in supplemental Table 1.
Histologic staining
The livers, spleens, and femurs from untreated or RN-1–treated SCD mice were removed immediately postmortem and weighed. Tissues from these organs were fixed, embedded, sectioned, and stained with hematoxylin-eosin (H&E).34,35 Semiquantification of histologic changes was analyzed as described in the supplemental Methods.
Immunoblotting
Protein extracts from control or RN-1–treated murine erythroleukemia (MEL) cells or SCD mice BM cells were subjected to western blotting as described,35 using the same antibodies against LSD1, H3K4me2, H3K9me2, and H3, as well as antibodies against PGC-1α and β-actin (Santa Cruz Biotechnology).
Cell culture and virus infection
Lineage-marker-negative (Lin–) BM cells from SCD mice were cultured and induced to undergo terminal erythroid differentiation.40,41 After 1 day of culture, Lin– cells were infected with an adenovirus that expressed PGC-1α (Ad-PGC-1α) or GFP (Ad-GFP) as a control.42 Infected cells were harvested at the indicated times for QRT-PCR, western blot, and flow cytometric analyses.
Results
Induction of HbF by RN-1 in SCD mice
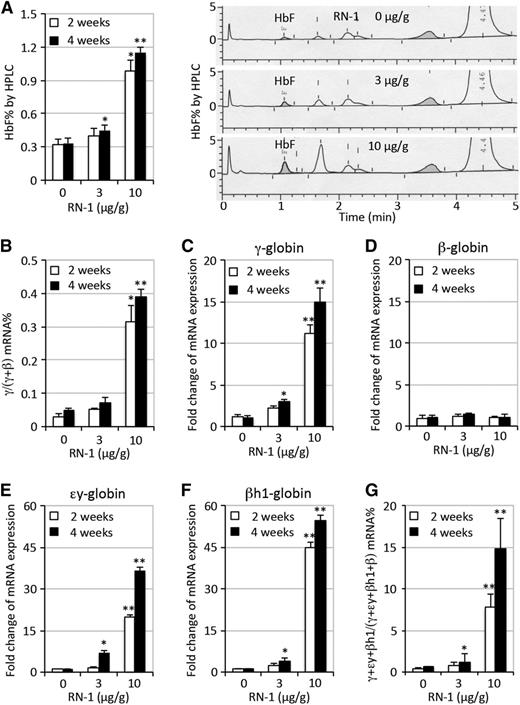
To determine whether RN-1 exerts in vivo effects on HbF induction in RBC progenitors, we intraperitoneally administered RN-1 daily (3 or 10 μg/g body weight/day) to SCD mice for 2 or 4 consecutive weeks. HbF induction was then determined by high-performance liquid chromatography (HPLC). In untreated SCD mice, HbF comprised an average of 0.32% of total hemoglobin, whereas in SCD mice exposed to the 3 μg RN-1/g body weight per day (hereafter referred to as the low RN-1 dosage), there was a statistically significant but modest (0.45%) HbF induction after 4 weeks of treatment. However, SCD mice treated with higher RN-1 dosage (10 μg/g) had a three to fourfold HbF induction (to 0.98% or 1.2%) after a 2- or 4-week drug exposure (Figure 1A).
Induction of HbF in SCD mice treated with RN-1. (A) Quantification of HbF by HPLC as a fraction of total hemoglobin in SCD mice either treated or untreated with RN-1 for 2 or 4 weeks (left panel); representative HPLC chromatograms depicting HbF abundance (shaded area) after 4 weeks RN-1 treatment (right panel). Other unmarked induced peaks may represent admixtures of murine embryonic εy and/or βh1 tetrameric hemoglobins (see below). (B) QRT-PCR quantification of γ-globin mRNA abundance as a fraction of the total of γ- plus β-globin mRNAs in SCD mice with or without RN-1 treatment of 2 and 4 weeks. (C-F) Fold change in the relative abundance of γ-globin mRNAs, normalized to the erythroid differentiation-invariant housekeeping mRNA, Oaz1, respectively.39 (G) QRT-PCR quantification of γ-, εy-, and βh1-globin mRNA abundance as a fraction of the total (γ-, εy- and βh1- plus β-globin mRNAs) in SCD mice with or without RN-1 treatment of 2 or 4 weeks. Statistically significant differences between RN-1–treated and untreated SCD mice are indicated (*P < .05; **P < .01). The bar graph data are presented as mean ± standard deviation (SD) (n = 4-6 mice per group).
Induction of HbF in SCD mice treated with RN-1. (A) Quantification of HbF by HPLC as a fraction of total hemoglobin in SCD mice either treated or untreated with RN-1 for 2 or 4 weeks (left panel); representative HPLC chromatograms depicting HbF abundance (shaded area) after 4 weeks RN-1 treatment (right panel). Other unmarked induced peaks may represent admixtures of murine embryonic εy and/or βh1 tetrameric hemoglobins (see below). (B) QRT-PCR quantification of γ-globin mRNA abundance as a fraction of the total of γ- plus β-globin mRNAs in SCD mice with or without RN-1 treatment of 2 and 4 weeks. (C-F) Fold change in the relative abundance of γ-globin mRNAs, normalized to the erythroid differentiation-invariant housekeeping mRNA, Oaz1, respectively.39 (G) QRT-PCR quantification of γ-, εy-, and βh1-globin mRNA abundance as a fraction of the total (γ-, εy- and βh1- plus β-globin mRNAs) in SCD mice with or without RN-1 treatment of 2 or 4 weeks. Statistically significant differences between RN-1–treated and untreated SCD mice are indicated (*P < .05; **P < .01). The bar graph data are presented as mean ± standard deviation (SD) (n = 4-6 mice per group).
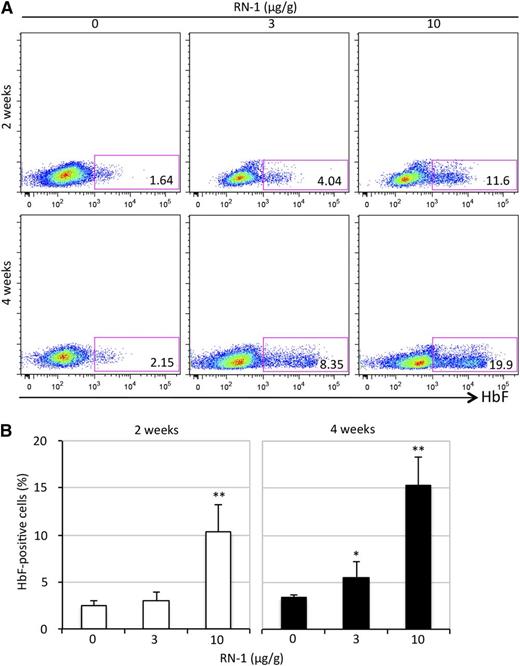
We then quantified γ-globin mRNA abundance by QRT-PCR. The relative γ-globin mRNA levels, when normalized to total human β-type globin (γ + β) transcripts, increased by more than an order of magnitude in SCD mice after 2 weeks (from 0.03% to 0.3%) or 4 weeks (to 0.4%) of RN-1 treatment at 10 μg/g (Figure 1B). To ascertain that the observed increase in γ-globin mRNAs was not an artifact attributed to normalizing against (γ + β) transcripts, a housekeeping mRNA Oaz1 that does not change in absolute abundance during erythroid differentiation was used as a second normalization control.39 γ-globin expression increased to two or 11-fold (at 2 weeks) and three- or 15-fold (at 4 weeks) when SCD mice were treated with either 3 or 10 μg/g of RN-1, respectively (Figure 1C). Adult β-globin transcription was unaffected by RN-1 administration (Figure 1D). Interestingly, we found that the murine εy- and βh1-globin mRNAs (homologs of the human ε- and γ-globin genes) increased to sevenfold and fourfold, respectively, after 4 weeks of 3 μg/g RN-1 treatment. However, much more robust increases were observed in the mouse embryonic εy- (20- or 36-fold) and βh1-globin (45- or 54-fold) mRNAs after 2 or 4 weeks, respectively, of 10 μg/g RN-1 exposure (Figure 1E-F). Furthermore, the percentage of human fetal γ- plus murine embryonic εy- and βh1-globin mRNAs, all of whose promoters bind to the TR2/TR4 core repressor complex, increased to 8% (at 2 weeks) or 15% (at 4 weeks) of total after 10 μg/g RN-1 treatment (Figure 1G). Flow cytometric analysis using an anti-HbF antibody showed that the HbF-high cell population was induced (to 10% to 15% of total) in SCD mice treated with higher RN-1 dosages after 2 or 4 weeks, respectively, indicating that the HbF-inducing effect was RN-1 concentration–dependent (Figure 2).
Flow cytometric analysis of HbF synthesis in circulating RN-1–treated RBCs. (A) Whole blood from SCD mice was stained with anti-human HbF antibody. The number in the rectangle of each representative sample shows the percentage of HbF-high cells within the total. (B) Statistical analysis of the percentage of HbF-high cells by flow cytometry averaged over all samples. Statistically significant differences between RN-1–treated and untreated SCD mice are indicated (*P < .05; **P < .01). Bar graph data are presented as the mean ± SD (n = 4-6 mice per group).
Flow cytometric analysis of HbF synthesis in circulating RN-1–treated RBCs. (A) Whole blood from SCD mice was stained with anti-human HbF antibody. The number in the rectangle of each representative sample shows the percentage of HbF-high cells within the total. (B) Statistical analysis of the percentage of HbF-high cells by flow cytometry averaged over all samples. Statistically significant differences between RN-1–treated and untreated SCD mice are indicated (*P < .05; **P < .01). Bar graph data are presented as the mean ± SD (n = 4-6 mice per group).
We also tested whether RN-1 could augment γ-globin synthesis in human primary erythroid cells that were differentiated ex vivo from mobilized CD34+ progenitor cells.29 The inductive effect of RN-1 (0.5 μM) on γ-globin accumulation was superior to HU (10 μM) or TC (0.5 μM), and was comparable with DAC (0.5 μM). The addition of RN-1 to HU, TC, or DAC exhibited no synergistic induction (supplemental Figure 1).
Effects of RN-1 on hematologic parameters of SCD mice
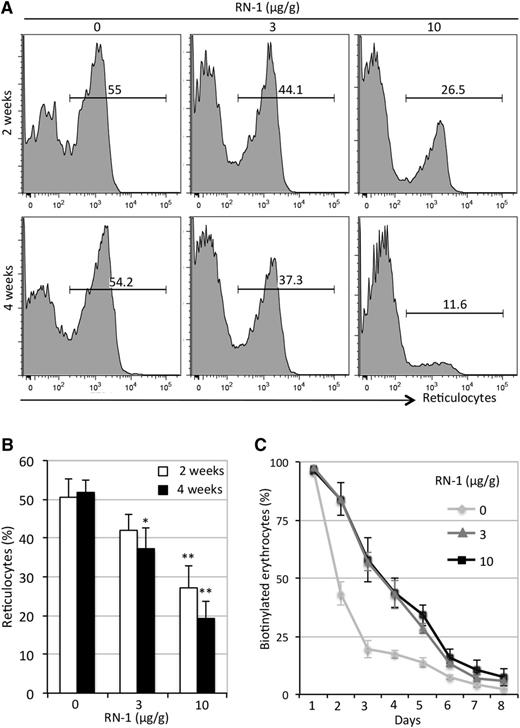
SCD mice have high reticulocyte counts (usually >50%), which is a sure sign of premature RBC destruction (hemolysis). We enumerated the reticulocyte in the peripheral blood of RN-1–treated or untreated SCD mice by flow-cytometric analyses after thiazole orange staining.34 Reticulocyte counts in RN-1–treated animals showed noticeable decreases (to 44% at 3 μg/g or 26% at 10 μg/g and to 37% at 3 μg/g or 12% at10 μg/g) after 2 and 4 weeks, respectively (Figure 3A-B). We further found that the lifespan of RBCs in RN-1–treated SCD mice increased from 2 to 5 days after treatment (Figure 3C).
Reduction in reticulocyte number and increased lifespan in RN-1–treated SCD mice. (A) The percentage of reticulocytes was measured by flow cytometry after thiazole orange staining of whole blood. The number shown above the horizontal bar in each box represents the fractional percentage of reticulocytes among the total red blood cells in each sample. (B) Statistical analysis of the percentage of reticulocytes in all samples from untreated and RN-1–treated SCD mice. Data are presented as mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01 vs untreated SCD mice). (C) Lifespan of erythroid cells in untreated or RN-1–treated SCD mice (data are depicted as the mean ± SD, n = 3 mice per group).
Reduction in reticulocyte number and increased lifespan in RN-1–treated SCD mice. (A) The percentage of reticulocytes was measured by flow cytometry after thiazole orange staining of whole blood. The number shown above the horizontal bar in each box represents the fractional percentage of reticulocytes among the total red blood cells in each sample. (B) Statistical analysis of the percentage of reticulocytes in all samples from untreated and RN-1–treated SCD mice. Data are presented as mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01 vs untreated SCD mice). (C) Lifespan of erythroid cells in untreated or RN-1–treated SCD mice (data are depicted as the mean ± SD, n = 3 mice per group).
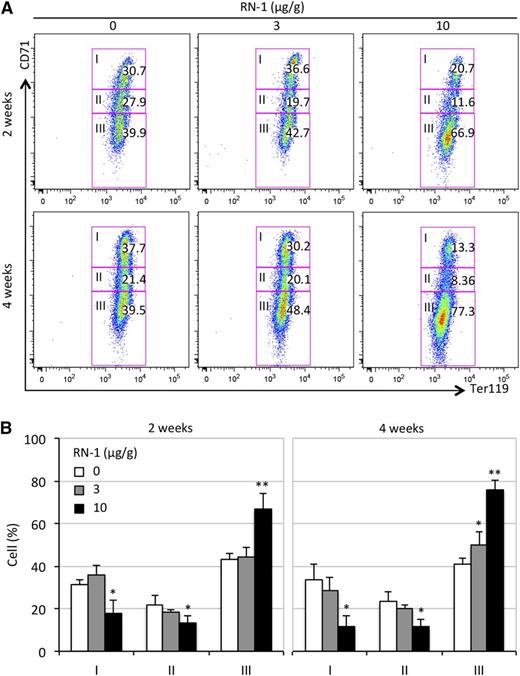
During erythroid differentiation, transferrin receptor CD71 expression precedes that of the erythroid-specific marker, Ter119. As differentiation progresses, CD71 expression is gradually extinguished.40 Using flow-cytometric assays, we analyzed the distribution of 3 erythroid differentiation stages (I ∼ III) of circulating RBCs in control and drug-treated SCD mice. Compared with untreated SCD mice (with ∼40% of mature CD71–Ter119+ erythroid cells, stage III), the increase in mature CD71–Ter119+ erythroid cells (43% or 67%) correlated with the given RN-1 dosages (3 or 10 μg/g), respectively, after 2 weeks (Figure 4 A-B). Four weeks after treatment had commenced, we saw a further increase (to 48% at lower dose or 77% at higher dose) in the CD71–Ter119+ erythroid cell fraction (Figure 4 A-B). In contrast, the fractions of immature CD71+Ter119+ (stage I) or intermediate CD71loTer119+ (stage II) erythroid cells were most noticeably reduced in SCD mice treated with the higher RN-1 dosage at the 2- and 4-week time points (Figure 4).
Flow cytometric assessment of erythroid cell differentiation in SCD mice treated with RN-1. (A) Peripheral blood cells were stained with anti-mouse CD71 and Ter119 antibodies to assess the erythroid differentiation profiles of RBCs in RN-1–treated or untreated SCD mice. Stained cells were sorted into 3 stages (I, immature; II, maturing; III, mature).40 The numbers in each rectangle represent the fractional percentages of cells at that developmental stage. (B) Statistically significant differences between differentiation stages of erythroid cells are indicated in comparing RN-1–treated or untreated SCD mice; data are presented as mean ± SD, n = 4-6 mice per group (*P < .05; **P < .01).
Flow cytometric assessment of erythroid cell differentiation in SCD mice treated with RN-1. (A) Peripheral blood cells were stained with anti-mouse CD71 and Ter119 antibodies to assess the erythroid differentiation profiles of RBCs in RN-1–treated or untreated SCD mice. Stained cells were sorted into 3 stages (I, immature; II, maturing; III, mature).40 The numbers in each rectangle represent the fractional percentages of cells at that developmental stage. (B) Statistically significant differences between differentiation stages of erythroid cells are indicated in comparing RN-1–treated or untreated SCD mice; data are presented as mean ± SD, n = 4-6 mice per group (*P < .05; **P < .01).
Complete blood cell counts performed on RN-1–treated mice showed that they had slightly elevated numbers of RBCs, more hemoglobin (HGB), elevated hematocrit (HCT), and white blood cell count (WBC), including lymphocytes, but lower platelets (PLT) and neutrophils (Neut), which were elevated in SCD mice when compared with wild-type mice (Table 1).43 In addition, the RBC distribution width (RDW) was significantly reduced after RN-1 treatment, suggesting that the size of circulating RBCs was more uniform in treated SCD compared with untreated mice (Table 1).
Hematologic parameters of SCD mice treated with RN-1
| . | . | 2 weeks . | . | . | 4 weeks . | . |
|---|---|---|---|---|---|---|
| RN-1 (μg/g) | 0 | 3 | 10 | 0 | 3 | 10 |
| RBC (× 106 cells/μL) | 7.15 ± 1.89 | 7.32 ± 0.69 | 8.02 ± 0.67 | 7.52 ± 0.90 | 7.66 ± 1.09 | 8.00 ± 2.21 |
| HGB (g/dL) | 5.00 ± 1.82 | 5.60 ± 0.89 | 6.00 ± 0.71 | 5.50 ± 0.70 | 6.00 ± 1.22 | 6.33 ± 1.52 |
| HCT (%) | 33.8 ± 8.95 | 38.00 ± 2.91 | 38.80 ± 3.03 | 38.70 ± 9.71 | 37.2 ± 4.32 | 37.5 ± 3.11 |
| MCV (fL) | 47.13 ± 1.72 | 51.92 ± 3.51* | 48.14 ± 0.78 | 48.93 ± 3.79 | 49.84 ± 4.91 | 45.84 ± 1.99 |
| MCH (pg) | 7.18 ± 0.54 | 7.70 ± 0.78 | 7.66 ± 0.52 | 8.27 ± 0.55 | 7.92 ± 0.51 | 7.08 ± 0.66 |
| MCHC (g/dL) | 15.18 ± 1.04 | 14.88 ± 1.55 | 15.92 ± 1.07 | 16.97 ± 1.69 | 16.06 ± 1.72 | 15.46 ± 0.92 |
| RDW (%) | 31.37 ± 1.10 | 27.64 ± 1.37† | 27.06 ± 2.59† | 31.20 ± 0.57 | 27.32 ± 0.97† | 25.10 ± 2.62† |
| PLT (× 103 cells/μL) | 1240 ± 142.40 | 528 ± 77.91† | 512 ± 88.71† | 1246.7 ± 259.29 | 858 ± 239.93* | 654 ± 274.65* |
| WBC (× 103 cells/μL) | 22.55 ± 8.78 | 20.78 ± 7.81 | 29.82 ± 11.56 | 26.40 ± 7.13 | 25.66 ± 10.15 | 31.06 ± 3.84 |
| Neut (× 103 cells/μL) | 2.63 ± 1.82 | 1.04 ± 0.69 | 1.20 ± 0.72 | 3.2 ± 1.25 | 1.98 ± 1.17 | 1.40 ± 0.73 |
| Lymph (× 103 cells/μL) | 18.53 ± 6.29 | 18.20 ± 6.56 | 26.74 ± 10.44 | 21.33 ± 5.62 | 21.76 ± 8.11 | 26.98 ± 2.06 |
| Mono (× 103 cells/μL) | 0.25 ± 0.17 | 0.30 ± 0.28 | 0.38 ± 0.16 | 0.23 ± 0.15 | 0.34 ± 0.27 | 0.20 ± 0.07 |
| Eos (× 103 cells/μL) | 0.48 ± 0.21 | 0.30 ± 0.14 | 0.32 ± 0.15 | 0.63 ± 0.15 | 0.66 ± 0.34 | 0.42 ± 0.11 |
| . | . | 2 weeks . | . | . | 4 weeks . | . |
|---|---|---|---|---|---|---|
| RN-1 (μg/g) | 0 | 3 | 10 | 0 | 3 | 10 |
| RBC (× 106 cells/μL) | 7.15 ± 1.89 | 7.32 ± 0.69 | 8.02 ± 0.67 | 7.52 ± 0.90 | 7.66 ± 1.09 | 8.00 ± 2.21 |
| HGB (g/dL) | 5.00 ± 1.82 | 5.60 ± 0.89 | 6.00 ± 0.71 | 5.50 ± 0.70 | 6.00 ± 1.22 | 6.33 ± 1.52 |
| HCT (%) | 33.8 ± 8.95 | 38.00 ± 2.91 | 38.80 ± 3.03 | 38.70 ± 9.71 | 37.2 ± 4.32 | 37.5 ± 3.11 |
| MCV (fL) | 47.13 ± 1.72 | 51.92 ± 3.51* | 48.14 ± 0.78 | 48.93 ± 3.79 | 49.84 ± 4.91 | 45.84 ± 1.99 |
| MCH (pg) | 7.18 ± 0.54 | 7.70 ± 0.78 | 7.66 ± 0.52 | 8.27 ± 0.55 | 7.92 ± 0.51 | 7.08 ± 0.66 |
| MCHC (g/dL) | 15.18 ± 1.04 | 14.88 ± 1.55 | 15.92 ± 1.07 | 16.97 ± 1.69 | 16.06 ± 1.72 | 15.46 ± 0.92 |
| RDW (%) | 31.37 ± 1.10 | 27.64 ± 1.37† | 27.06 ± 2.59† | 31.20 ± 0.57 | 27.32 ± 0.97† | 25.10 ± 2.62† |
| PLT (× 103 cells/μL) | 1240 ± 142.40 | 528 ± 77.91† | 512 ± 88.71† | 1246.7 ± 259.29 | 858 ± 239.93* | 654 ± 274.65* |
| WBC (× 103 cells/μL) | 22.55 ± 8.78 | 20.78 ± 7.81 | 29.82 ± 11.56 | 26.40 ± 7.13 | 25.66 ± 10.15 | 31.06 ± 3.84 |
| Neut (× 103 cells/μL) | 2.63 ± 1.82 | 1.04 ± 0.69 | 1.20 ± 0.72 | 3.2 ± 1.25 | 1.98 ± 1.17 | 1.40 ± 0.73 |
| Lymph (× 103 cells/μL) | 18.53 ± 6.29 | 18.20 ± 6.56 | 26.74 ± 10.44 | 21.33 ± 5.62 | 21.76 ± 8.11 | 26.98 ± 2.06 |
| Mono (× 103 cells/μL) | 0.25 ± 0.17 | 0.30 ± 0.28 | 0.38 ± 0.16 | 0.23 ± 0.15 | 0.34 ± 0.27 | 0.20 ± 0.07 |
| Eos (× 103 cells/μL) | 0.48 ± 0.21 | 0.30 ± 0.14 | 0.32 ± 0.15 | 0.63 ± 0.15 | 0.66 ± 0.34 | 0.42 ± 0.11 |
Eos, eosinocyte; HCT, hematocrit; HGB, hemoglobin; Lymph, lymphocyte; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; Mono, monocyte; Neut, neutrophil; PLT, platelet; RBC, red blood cell; RDW, red blood cell distribution width; WBC, white blood cell.
P < .05.
P < .01 vs untreated SCD mice.
Taken together, these studies showed that first, repeated daily administration of RN-1 led to improved half-life and indices (cell number and size, hemoglobin content, hematocrit) of RBCs, and consequently reduced hemolysis (and therefore, the reticulocyte count) and higher fraction of circulating mature erythrocytes in SCD mice. Second, daily injections of RN-1 (even at the higher dose [10 μg/g body weight/day]) did not have serious adverse effect on other hematopoietic cell lineages.
Effects of RN-1 on pathohistologic characteristics of SCD mice
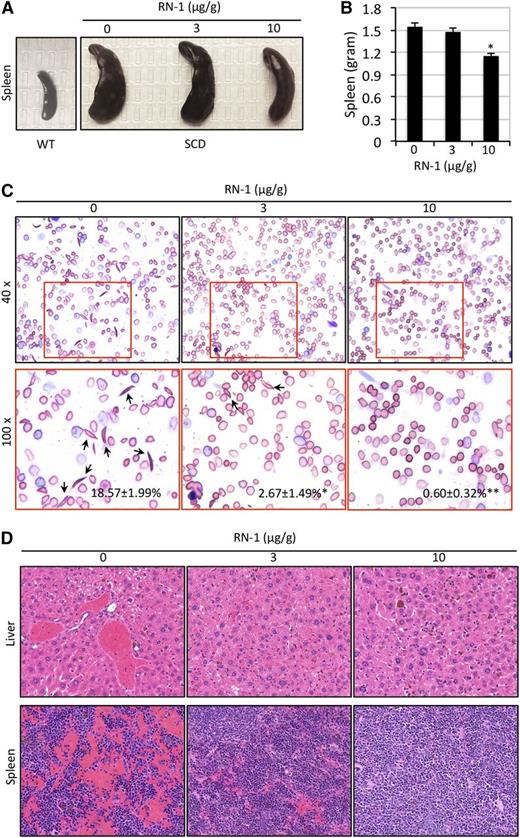
To evaluate whether RN-1 could reverse the pathophysiologic symptoms (hepatosplenomegaly and organ necrosis) that are usually associated with SCD, mice were sacrificed after 4 weeks of RN-1 treatment and their livers and spleens were weighed. In contrast to the spleen size of untreated SCD mice, animals treated with higher dosage of RN-1 had a statistically significant reduction in spleen size, whereas SCD mice treated with lower RN-1 dosage exhibited only modest effects on splenomegaly (Figure 5A-B). There was no significant difference between the liver sizes of untreated and treated SCD mice (data not shown).
Reduced organ damage and RBC sickling in SCD mice treated with RN-1. (A-B) SCD mice treated with RN-1 exhibited modestly reduced splenomegaly. The size (A) and the weight (B) of spleens are depicted (*P < .05 vs untreated SCD mice). (C) Wright-Giemsa staining of peripheral blood smears of SCD mice before and after daily RN-1 administration for 1 month. Arrows indicate sickled cells. The average percentage of sickle cells in each group is presented as the mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01). (D) H&E staining of liver and spleen sections from untreated or RN-1–treated SCD mice.
Reduced organ damage and RBC sickling in SCD mice treated with RN-1. (A-B) SCD mice treated with RN-1 exhibited modestly reduced splenomegaly. The size (A) and the weight (B) of spleens are depicted (*P < .05 vs untreated SCD mice). (C) Wright-Giemsa staining of peripheral blood smears of SCD mice before and after daily RN-1 administration for 1 month. Arrows indicate sickled cells. The average percentage of sickle cells in each group is presented as the mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01). (D) H&E staining of liver and spleen sections from untreated or RN-1–treated SCD mice.
Erythroid cell morphology was evaluated by staining blood smears prepared from untreated and RN-1–treated SCD mice with Wright-Giemsa stain. The number of sickled RBCs was significantly reduced in SCD mice treated with RN-1 for 4 weeks at both dosages (Figure 5C). This is consistent with our earlier observation that the RDW was the same in SCD mice treated with the low or high RN-1 dose (Table 1). Hence, it appears that RN-1 can inhibit RBC sickling, thereby prolonging the lifespan of RBCs (Figure 3C), consequently resulting in increased accumulation of mature CD71–Ter119+ erythroid cells (Figure 4) in the circulation of treated mice.
We further examined the pathophysiologic characteristics of RN-1–treated (or untreated) SCD mice in tissue sections. After 4 weeks, the livers, spleens, and femurs from the SCD mice were removed and processed for histologic analyses. Upon examination, although substantial necrosis in the livers and spleens of untreated SCD control mice was observed, there was milder or virtually complete absence of tissue necrosis in SCD mice treated with RN-1 (Figure 5D). Semiquantitative analysis of necrosis in histologic specimens of the livers and spleens indicated that the pathologic changes in RN-1–treated SCD mice were significantly improved compared with untreated SCD animals (supplemental Figure 2A-B). Examination of histologic sections of the femurs of untreated SCD mice showed high marrow cellularity, probably because of increased erythropoiesis. In contrast, the marrow of treated SCD animals displayed markedly reduced hypercellularity in response to RN-1 treatment (supplemental Figure 3).
We also performed serum chemistry analysis including total bilirubin, lactate dehydrogenase, aspartate transaminase, and alanine transaminase (supplemental Figure 4A-D), as well as urinalysis of RN-1–treated and untreated SCD mice (supplemental Table 2). Diminished levels of total bilirubin, lactate dehydrogenase, aspartate transaminase, and alanine transaminase levels were observed in SCD mice treated with RN-1 in a dose- and time-dependent manner (supplemental Figure 4A-D), which is consistent with the reduced hemolysis and histologic improvements in RN-1–treated SCD mice. Detectable levels of protein and bilirubin in the urine of untreated SCD mice were cleared after 4 weeks of 10 μg/g RN-1 treatment, and there were no obvious treatment-related effects on other urinalysis parameters (supplemental Table 2). These observations indicate that RN-1 treatment of SCD mice leads to effective reversal of much of the disease pathology normally associated with SCD.
Effects of RN-1 on LSD1 binding to β-type globin gene promoters
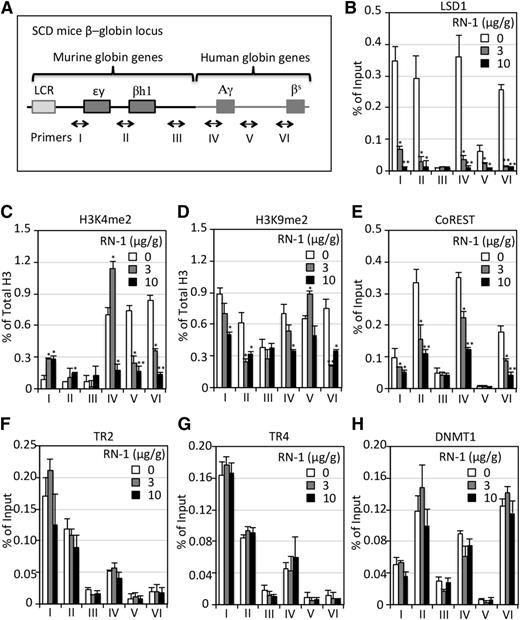
To determine whether RN-1 affects LSD1 binding to the β-type globin gene promoters in differentiating erythroid cells, we performed ChIP assays using BM cells derived from untreated or drug-treated SCD mice (Figure 6A). LSD1 binding to the human γ- and β-globin promoters and murine εy- and βh1-globin promoters was readily detected in the hematopoietic cells of untreated animals (Figure 6B). However, in RN-1–treated SCD mice, LSD1 binding to the promoter regions throughout the locus was diminished in a dose-dependent manner (Figure 6B). Consistent with the reduced binding of LSD1 to β-globin locus promoters, RN-1 treatment of SCD mice also led to decreased abundance of LSD1 protein in BM cells (supplemental Figure 5). Similar results were observed in cultured MEL cells treated with different concentrations of RN-1 in vitro (supplemental Figure 6).
ChIP analysis of epigenetic cofactor binding across the β-globin locus in SCD mice before and after RN-1 treatment. (A) Schematic diagram of the β-globin locus in SCD mice. A 9.7-kb DNA fragment containing the human Aγ-globin gene and human sickle β-globin (βS) gene replaced the mouse βmajor- and βminor-globin genes.33 Primers were designed to amplify discrete regions across the β-globin locus, including (I) the εy-globin promoter DR site, (II) the βh1-globin promoter DR site, (III) the βmajor-globin upstream 5.9 kbp (presumptive negative control, (IV) the Aγ-globin promoter DR site, (V) a +3-kb region downstream of the Aγ-globin gene, and (VI) the β-globin promoter CAAT box (precise positions of the primer pairs used for these assays are given in supplemental Table 1). (B-H) ChIP analyses of LSD1, H3K4me2, H3K9me2, CoREST, TR2, TR4, and DNMT1 binding to each region of the β-globin locus (A) in BM cells of SCD mice before or after RN-1 treatment. The level of H3K4me2 and H3K9me2 is normalized to the fraction of total histone 3, whereas the level of all other proteins is normalized to control IgG. Data are presented as mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01 vs untreated SCD mice).
ChIP analysis of epigenetic cofactor binding across the β-globin locus in SCD mice before and after RN-1 treatment. (A) Schematic diagram of the β-globin locus in SCD mice. A 9.7-kb DNA fragment containing the human Aγ-globin gene and human sickle β-globin (βS) gene replaced the mouse βmajor- and βminor-globin genes.33 Primers were designed to amplify discrete regions across the β-globin locus, including (I) the εy-globin promoter DR site, (II) the βh1-globin promoter DR site, (III) the βmajor-globin upstream 5.9 kbp (presumptive negative control, (IV) the Aγ-globin promoter DR site, (V) a +3-kb region downstream of the Aγ-globin gene, and (VI) the β-globin promoter CAAT box (precise positions of the primer pairs used for these assays are given in supplemental Table 1). (B-H) ChIP analyses of LSD1, H3K4me2, H3K9me2, CoREST, TR2, TR4, and DNMT1 binding to each region of the β-globin locus (A) in BM cells of SCD mice before or after RN-1 treatment. The level of H3K4me2 and H3K9me2 is normalized to the fraction of total histone 3, whereas the level of all other proteins is normalized to control IgG. Data are presented as mean ± SD (n = 4-6 mice per group) (*P < .05; **P < .01 vs untreated SCD mice).
In western blotting analyses, MEL cells treated with increasing concentrations of RN-1 (0 - 200 nM) showed a modest increase in H3K4me2 and H3K9me2 levels (supplemental Figure 6). Interestingly, immunoblotting analyses of BM cells of SCD mice showed that the H3K4me2 and H3K9me2 levels both increased when mice were treated with the lower dose, but not with the higher dose, of RN-1 (supplemental Figure 5). To assess potential off-target effects on those changes in methylation, we verified the published specificity of RN-1 against LSD1 and other lysine demethylases (KDMs), including KDM5B44 and KDM5C,45 KDM4A (JMJD2A),46 and KDM4D (JMJD2D).47 We confirmed that RN-1 is a potent and quite selective inhibitor of LSD1 when compared with the other tested KDMs (supplemental Figure 7).
To determine whether these global changes in H3 marks reflect β-type globin gene-specific activation or repression activity, we examined the effect of RN-1 treatment on the levels of H3K4me2 and H3K9me2 throughout the genetically modified β-globin gene locus in SCD mice. We found that the abundance of H3K4me2 (normalized to total H3) increased at the γ-, εy-, and βh1-globin promoters when SCD mice were treated with 3 μg/g RN-1 for 4 weeks, and that there was no further increase, but instead a sharp decrease, of H3K4me2 at the γ-globin promoter when treated with higher RN-1 concentration (Figure 6C), which correlated with the H3K4me2 levels shown by western blotting (supplemental Figure 5). The levels of H3K9me2 at both the human γ- and β-globin promoters and murine εy- and βh1-globin promoters were reduced when the SCD mice were treated with RN-1 for 4 weeks (Figure 6D).
We next examined the chromatin occupancy of several previously identified LSD1- interacting proteins, including CoREST, TR2, TR4, and DNMT1.25 RN-1 treatment markedly reduced the binding of CoREST (Figure 6E), but only slightly altered the binding of TR2, TR4, or DNMT1 within the β-globin locus (Figure 6F-H). The data indicated that LSD1 binding to the globin promoters disrupted only the complex association with CoREST, and did not affect the binding of other members of the quaternary “core” repression complex (TR2, TR4, DNMT1, and LSD1).25
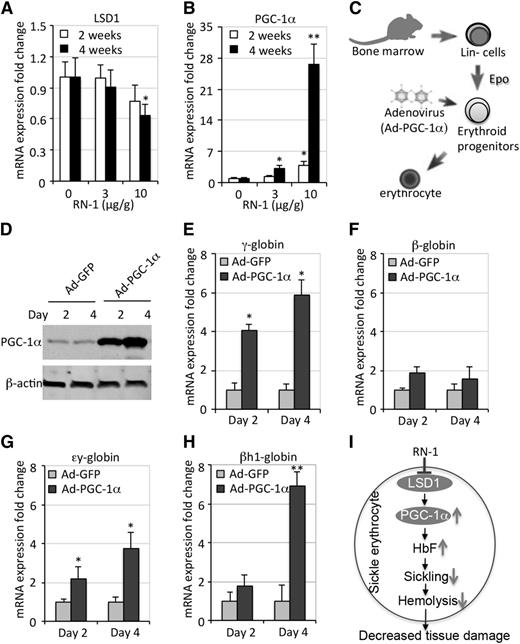
RN-1 induces fetal globin gene expression via upregulation of PGC-1α
To elucidate the molecular mechanisms underlying the in vivo HbF-inducing effects of RN-1, we next focused on LSD1 target genes that have been shown to be direct regulators of the globin genes. PPARγ coactivator-1α (PGC-1α) was previously identified as a LSD1 direct target gene.48 We recently showed that PGC-1α activity is required for both murine erythropoiesis as well as for globin gene regulation.35 To determine whether the induction of HbF in SCD mice by RN-1 treatment might be mediated by PGC-1α, we examined the expression of PGC-1α in SCD mice treated (or not) with RN-1. We discovered that the loss of LSD1 after RN-1 treatment in SCD mice increased H3K4me2 levels at the PGC-1α promoter (supplemental Figure 8A) and induced the expression of PGC-1α in a dose-dependent manner (Figure 7A-B), suggesting that elevated PGC-1α expression might contribute to the induction of the γ-globin gene in these mice.
RN-1 induces γ-globin synthesis by inducing PGC-1α. (A-B) QRT-PCR analyses quantify the fold change in LSD1 and PGC-1α mRNAs after normalization to the expression of Oaz1. (C) Diagrammatic representation of the gain-of-function PGC-1α experiment. Lin– BM cells from SCD mice were induced to undergo terminal erythroid differentiation in vitro after infection with adenoviruses that forcibly expressed PGC-1α (Ad-PGC-1α) or GFP (Ad-GFP). (D) Western blots depict abundant expression of PGC-1α in infected Lin– BM cells. (E-H) The relative fold change of γ-, β-, εy-, and βh1-globin mRNA abundances normalized to Oaz1 mRNA, respectively. (I) Schematic model depicting a simplified mechanism by which RN-1 may indirectly (as well as directly, Figure 6B) control fetal globin gene expression by inhibition of LSD1 activity.
RN-1 induces γ-globin synthesis by inducing PGC-1α. (A-B) QRT-PCR analyses quantify the fold change in LSD1 and PGC-1α mRNAs after normalization to the expression of Oaz1. (C) Diagrammatic representation of the gain-of-function PGC-1α experiment. Lin– BM cells from SCD mice were induced to undergo terminal erythroid differentiation in vitro after infection with adenoviruses that forcibly expressed PGC-1α (Ad-PGC-1α) or GFP (Ad-GFP). (D) Western blots depict abundant expression of PGC-1α in infected Lin– BM cells. (E-H) The relative fold change of γ-, β-, εy-, and βh1-globin mRNA abundances normalized to Oaz1 mRNA, respectively. (I) Schematic model depicting a simplified mechanism by which RN-1 may indirectly (as well as directly, Figure 6B) control fetal globin gene expression by inhibition of LSD1 activity.
To test this hypothesis, we isolated Lin– cells (containing a mixture of hematopoietic stem and progenitor cells) from the BM of SCD mice and cultured the cells in media supplemented with erythropoietin (Epo) to support terminal erythroid differentiation.40,41 PGC-1α was forcibly expressed in Lin– BM cells by infection with a PGC-1α–expressing adenovirus (Ad-PGC-1α) (Figure 7C-D). After several days, the cells were examined for mRNA expression of the globin genes. The data show that overexpressing PGC-1α resulted in increased human fetal γ-globin mRNA as well as more abundant expression of the murine embryonic εy- and βh1-globin genes, with only slightly increased adult β-globin mRNA levels (Figure 7E-H). We also found that PGC-1α forced expression in Lin– BM cells slightly improved cell maturation without affecting cell viability (supplemental Figure 8B). These results directly validate a role for PGC-1α in γ-globin induction and are consistent with the hypothesis that inhibition of LSD1 by RN-1 induces γ-globin synthesis by de-repressing the gene encoding the transcriptional coactivator PGC-1α (Figure 7I).
Discussion
We recently identified LSD1 as a new protein involved in the regulation of the fetal γ-globin genes.25 Inhibition of LSD1 by RNA interference or by the small-molecule inhibitor TC enhanced γ-globin expression in differentiated human CD34+ cells in vitro.30 Thus, the inhibition of LSD1 activity presents a novel exploratory avenue as a therapeutic strategy to treat SCD. However, TC treatment in patients was shown to have potentially lethal side effects that stem from interactions with specific natural metabolites,49 and at higher concentrations also resulted in decreased production of β-like globin mRNAs and impaired erythroid maturation.31 To develop safer and more beneficial therapeutics for the treatment of SCD patients, here we tested another LSD1 inhibitor, RN-1, which has no off-target effect directed against other demethylases (supplemental Figure 7).
As anticipated, RN-1 treatment of SCD mice induced γ-globin expression and HbF synthesis (Figure 1). The magnitude of γ-globin induction in the SCD mouse by RN-1 is comparable with that previously reported for decitabine or HU in transgenic mouse models.50,51 Notably, RN-1 treatment robustly increased the expression of murine embryonic εy- and βh1-globin genes (Figure 1E-F). The differential responses of the human and endogenous mouse embryonic globin genes to induction could be caused by an incomplete repertoire of human γ-globin regulatory elements within the modified murine β-globin locus substitutions. Unlike TC, RN-1 treatment at both low and high concentrations did not cause a reduction in adult β-globin expression or erythroid differentiation (Figure 1D).30,31
Clinical data indicate that induction of HbF to a three- to fivefold increase over the normal baseline in patients can significantly alleviate the clinical manifestations of SCD.3,4 The cumulative abundance of human and mouse early globin mRNAs maximally attained in SCD mice was 15% (Figure 1G) of all globin transcripts. Of greater relevance, RN-1 can induce HbF levels three- to fourfold above the baseline level in control SCD mice (Figure 1A). Hence, these results tantalizingly suggest that RN-1 or a close analog could be an effective therapeutic treatment of SCD patients. Our additional findings showed that reduced hemolysis, as demonstrated by reduced reticulocyte counts (Figure 3A), decreased total bilirubin (supplemental Figure 3A), decreased sickling (Figure 5C), and prolonged the lifespan of RBCs (Figure 3C) in SCD mice treated with RN-1, led to an increased number of mature circulating erythrocytes (Figure 4) and hemoglobin content (Figure 1A and Table 1) as well as improved organ pathophysiology (Figure 5D and supplemental Figures 2 and 3). Higher doses (10 μg/g) of RN-1 yielded significantly more robust therapeutic effects than the lower dosage examined here (3 μg/g) in SCD mice, possibly because the higher dose regimen results in a serum concentration that is twofold higher than the IC50, whereas the serum concentration at the lower dose regimen is below the IC50.32 These findings provide ample evidence for the hematologic and physiologic benefits of interfering with LSD1 by administration of RN-1 in SCD mice and clearly indicates that RN-1 or its derivatives might be effective in the treatment of SCD as novel HbF-inducing agents.
ChIP analysis confirmed that LSD1 is bound to the promoter of the γ-globin gene in SCD mice, and that RN-1 treatment decreased the promoter occupancy of LSD1 (Figure 6B). This was also accompanied by a decrease in dimethylation of H3K9 (Figure 6D) as well as an increase in dimethylation of H3K4 (only seen at the lower concentration of RN-1) (Figure 6C). Unexpectedly, after increasing the concentration of RN-1, there was no further enhancement of (activating) H3K4me2 accumulation at the γ-globin promoter (Figure 6C). This may be the result of a compensatory effect by other H3K4 demethylases, because RN-1 does not affect the activity of other KDMs (supplemental Figure 7). These results indicate that one possible mechanism for γ-globin induction by RN-1 is a result of reduced H3K9me2 at its promoter as a consequence of LSD1 inhibition or loss. However, because a growing number of nuclear receptors as well as other transcription factors have been shown to associate with LSD1, these effects could be mediated through any of these other DNA-binding proteins.25,31 The ChIP analyses reported here showed that the chromatin occupancy of one LSD1 cofactor, CoREST, within the β-globin locus is dependent on the presence of LSD1 in the BM cells of SCD mice (Figure 6E). In contrast, the binding of several other known LSD1-interacting proteins, including TR2, TR4, and DNMT1,27 appear to be unaltered upon LSD1 loss after RN-1 treatment (Figure 6F-H).
Genome-wide analyses of LSD1-regulated genes revealed that PGC-1α is a direct target gene of LSD1, and loss of LSD1 function in adipocytes was shown to induce the expression of PGC-1α.48 We recently showed that PGC-1α is also required for murine erythropoiesis and globin gene regulation, and that PGC-1α knock-out mice exhibited a significant reduction in the embryonic εy-and βh1-globin mRNAs when compared with wild-type mice.35 It seemed possible that the inhibition of LSD1 by RN-1 in SCD murine erythroid cells might induce PGC-1α expression, resulting in the activation of fetal γ-globin and embryonic εy-and βh1-globin genes, and the data shown here confirmed that hypothesis. Although the level of LSD1 decreased upon continuous RN-1 treatment, the abundance of PGC-1α transcript markedly increased in erythrocytes (Figure 7A-B). We further found that overexpression of PGC-1α by adenovirus infection of Lin– BM cells followed by differentiation into erythrocytes (Figure 7C-D) led to increased expression of both the (human) fetal γ-globin and (murine) embryonic εy-and βh1-globins (Figure 7E-H), which phenocopied the results of RN-1 administration in SCD mice (Figure 1). These in vivo and in vitro data support a novel model in which PGC-1α induction (attributed to LSD1 inactivation) contributes to increases in HbF in erythrocytes, which in turn leads to reduced sickling, hemolysis, and tissue injury in SCD mice (Figure 7I). In conclusion, these studies not only lend further support to the growing body of evidence that interfering with LSD1 activity may be an effective approach that could therapeutically benefit individuals with SCD, but also identified PGC-1α as a potential link in the human globin gene regulatory framework.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to their colleagues for many insightful comments on the manuscript.
This work was supported by an American Heart Association Scientist Development grant (S.C.), and by the National Institutes of Health, National Heart, Lung and Blood Institute research grants R01HL24415 and U01HL117658 (J.D.E.).
Authorship
Contribution: S.C. designed, performed, and analyzed experiments and wrote the paper; K.-C.L. analyzed experiments and wrote the paper; M.L., N.J., G.M., Y.S., and L.S. performed experiments; D.H. performed and analyzed experiments; A.C., A.R., J.D., and D.L. commented on and analyzed experiments; S.I. and R.C.T. contributed vital reagents and wrote the paper; and J.D.E. designed and analyzed experiments and wrote the paper.
Conflict-of-interest disclsoure: The authors declare no competing financial interests.
Correspondence: James Douglas Engel, University of Michigan, 3072 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200; e-mail: engel@umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal