In this issue of Blood, under the unassuming title “Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency,” Pike et al1 publish observations on a very rare condition, but the results validate the real-life importance of a scheme of thrombin generation that has been emerging from biochemical research over the last decades and that challenges such stereotypes as the “clotting cascade” and “primary and secondary hemostasis.” Moreover, this article shows how a bleeding phenotype is best recognized in the laboratory.
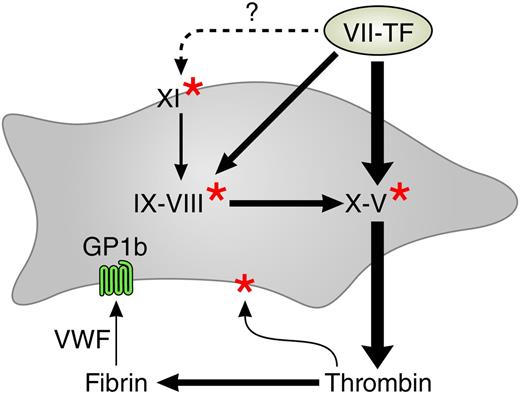
The procoagulant network. Arrows indicate activations. The thicker arrows are operative at higher tissue factor concentrations. Red stars indicate activation by thrombin. The gray surface represents the platelet, and the light green surface represents tissue factor–containing membrane from wounded tissue, microparticles, or blood-borne tissue factor. VWF, von Willebrand factor. The dotted arrow denotes unconfirmed tissue factor–mediated activation of factor XI.
The procoagulant network. Arrows indicate activations. The thicker arrows are operative at higher tissue factor concentrations. Red stars indicate activation by thrombin. The gray surface represents the platelet, and the light green surface represents tissue factor–containing membrane from wounded tissue, microparticles, or blood-borne tissue factor. VWF, von Willebrand factor. The dotted arrow denotes unconfirmed tissue factor–mediated activation of factor XI.
An important physiologic function in hemostasis and thrombosis—if not the most important—is the amount of thrombin that blood can provide at the site of a lesion. In hemophilia and pharmacologic anticoagulation, the capacity to form thrombin is diminished, and the risk of bleeding increases as the amount of thrombin decreases. To estimate the thrombin-generating capacity, 2 approaches are used: the chemical and the physiologic. Chemical approaches evaluate clotting factors or the genes that govern their synthesis and infer the thrombin generation capacity via our knowledge of the role of these factors in the clotting mechanism. Physiologic approaches judge the amount of thrombin that blood can form by determining a clotting time or by more sophisticated methods. Such tests are fundamentally different from the chemical type in that they are essentially function tests of an isolated organ (ie, blood, platelet-rich plasma [PRP] or platelet-poor plasma [PPP]).
Strangely enough, even in the hemophilias, which are evidently one gene–one protein diseases, no one-to-one relationship is found between the bleeding phenotype and the level of the deficient factor (VIII, IX, XI) and/or the type of underlying genetic defect1,2 (also see references in Pike et al1 ). Apparently, some patients can make better use of the same small amount of residual factor than others. The article of Pike et al shows that the physiologic approach can yield the information about the bleeding phenotype that the chemical approach has not been able to provide, under the proviso that the test on the isolated sample faithfully represents the function of the organ in vivo.
The activated partial thromboplastin time does not serve well as a function test because it is entirely dependent on contact activation, which, as Pike et al show, is precisely the process that supersedes the function of factor XI (FXI) in the role in which it prevents bleeding in patients. Furthermore, any clotting time informs about the initiation phase of thrombin formation only because plasma clots as soon as ∼1% of all thrombin is formed.
The last decade has seen a revival of the measurement of thrombin over the complete course of its formation and inactivation, facilitated by the development of methods that allow easy registration of the thrombin generation curve.3 Numerous studies—too many to be adequately referenced here—can be summarized as “The less thrombin, the more bleeding.” This has been shown to hold in hemophilia A and B, in a number of rare clotting-factor deficiencies, and in pharmacologic anticoagulation. Pike et al show that it holds for FXI deficiencies as well, provided that that the test is carried out in PRP and that contact activation is inhibited. Apparently, platelets form such an integral part of the clotting system that its function cannot be adequately judged from PPP alone in all instances.4
The importance of platelets in “secondary hemostasis” became apparent in 19875 and is strongly supported by the experiments leading to the concept of cell-mediated coagulation.6 Together with the findings of the Furie group,7 which demonstrate a role of thrombin in the first seconds after a vessel wall lesion occurs (ie, in “primary hemostasis”), this defies the very concept of primary and secondary hemostasis.
Why Pike et al find thrombin generation in PRP to be more informative than that in PPP is not yet completely understood. We know that the scrambling of the platelet membrane upon activation provides the necessary procoagulant phospholipids8 and that they release factor V. The role of platelet-FXI interaction has been and still is extensively studied and there has yet to be a final word (see Walsh and Galiani9 and Walsh10 for references). What we learn from the present article is that a model of thrombin generation that explains bleeding in clinical reality should include the platelet and should exclude factor XII (see figure).
In such a model, the triggering of the procoagulant activities of the platelets is an integral part. We know that at least 3 mechanisms play a role: (1) the direct activation by thrombin via the PAR1 and PAR4 receptors, (2) the activation of platelet receptor GP1b by von Willebrand factor that incorporates in the growing clot,11 and (3) the formation on the activated platelet of αIIβ3 receptors that interact with fibrinogen and further enhance membrane scrambling.
The scheme (see figure) that explains the findings of Pike et al no longer looks like a cascade with an intrinsic and an extrinsic pathway branch, but rather like a network replete with feedback activations. A complete diagram should also contain the antithrombins and the feedback inhibitions (proteins C and S, tissue factor pathway inhibitor, etc), which is beyond the scope of this commentary. There are also reports on tissue factor–mediated activation of factor XI but they have yet to be confirmed.
In conclusion, this article shows that the alternative scheme of coagulation that emerged from numerous biochemical studies (that alas cannot be adequately referenced here) seems to apply to clinical reality and that, not surprisingly, the clinical value of phenotyping the clotting system via its thrombin-generating capacity is more reliable when more elements of the in vivo system are included.
Conflict-of-interest disclosure: H.C.H. is a part-time employee of Synapse BV and a consultant to Diagnostica Stago (Asnières, France).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal