In this issue of Blood, Sayed et al report on a series of 53 patients with light chain (LC) deposition disease (LCDD) prospectively followed for a median of 6.2 years, showing divergent outcomes in patients who achieve a very good partial response (VGPR) or complete response (CR) compared with those who do not (see figure, panel A). Even patients with advanced chronic kidney disease (CKD) benefited from a deep clonal response.1
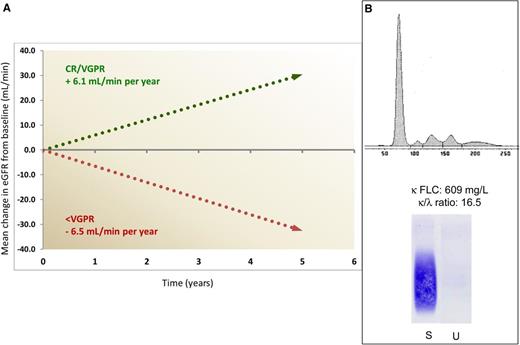
Impact of response on progression of renal damage and challenging electrophoretic pattern in light chain deposition disease. (A) Mean annual change in estimated glomerular filtration rate (eGFR) in patients attaining a VGPR or CR, compared with patients reaching less than VGPR. Only 14% of patients in VGPR or CR required dialysis, compared with 64% of other subjects. (B) The serum electrophoresis scan (top) and anti-κ immunofixation (bottom) show no monoclonal immunoglobulin in a patient with LCDD and with a serum κ free LC (FLC) concentration of 609 mg/L and a greatly abnormal FLC ratio. In our population of 68 patients with LCDD, a monoclonal protein was detected by serum and urine electrophoresis/immunofixation in only 60% of the patients, whereas FLC concentration and ratio were abnormal in all. S, serum; U, urine.
Impact of response on progression of renal damage and challenging electrophoretic pattern in light chain deposition disease. (A) Mean annual change in estimated glomerular filtration rate (eGFR) in patients attaining a VGPR or CR, compared with patients reaching less than VGPR. Only 14% of patients in VGPR or CR required dialysis, compared with 64% of other subjects. (B) The serum electrophoresis scan (top) and anti-κ immunofixation (bottom) show no monoclonal immunoglobulin in a patient with LCDD and with a serum κ free LC (FLC) concentration of 609 mg/L and a greatly abnormal FLC ratio. In our population of 68 patients with LCDD, a monoclonal protein was detected by serum and urine electrophoresis/immunofixation in only 60% of the patients, whereas FLC concentration and ratio were abnormal in all. S, serum; U, urine.
LCDD is an ultra-rare condition caused by the deposition of monoclonal LCs in basement membranes. These LCs are produced by a small (∼90% of patients with <10% bone marrow plasma cells) yet extremely dangerous clone.2 As opposed to light chain (AL) amyloidosis, the LC is κ in ∼80% of patients. The affinity for basement membrane components is probably caused by the peculiar structural features of the LC, with somatic mutations resulting in the exposure of hydrophobic residues in complementarity determining regions 1 or 3, with an increased tendency to aggregate and/or to adhere to basement membranes.3,4 This interaction, leading to continued LC deposition and progressive organ damage, makes early diagnosis and the rapid and profound suppression of the production of the “toxic” LC essential to halt renal damage, favoring the catabolism of deposited LC and the recovery of organ function. LCDD belongs to the wide group of monoclonal gammopathies of renal significance5 because the main target organ is the kidney, with hematuria, hypertension, proteinuria, and, ultimately, end-stage renal disease. Overt involvement of other organs, such as liver, heart, lung, and nerves, is infrequent. The rarity of LCDD has so far hampered clinical studies on this condition, and our knowledge of the natural history of this disease and treatment outcomes is largely based on small retrospective case series.
Sayed and colleagues should be commended for reporting a large series of patients diagnosed after 2002 and systematically followed with measurements of FLCs and markers of organ damage. Their findings offer the opportunity to highlight 2 important points: (1) the vital importance of early diagnosis and (2) the high efficiency of bortezomib-based therapy in preserving and rescuing renal function.
They also report that almost one-fifth of patients were already on renal replacement therapy at diagnosis and 38% had severe or end-stage renal failure (CKD stage 4 or 5) that led to dialysis in a median time of 2.7 years, compared with 9.0 years for patients with moderately impaired renal dysfunction (CKD stage 2 or 3). This is in agreement with recent observations in 2 relatively large LCDD patient populations, with 16% to 23% of patients already on dialysis at diagnosis and mean serum creatinine concentration at diagnosis of 2.6 to 3.8 mg/dL.6,7 These findings demonstrate that even now, advanced renal dysfunction is common at diagnosis, with a major impact on renal outcomes. In fact, although chemotherapy can rescue patients even in advanced (CKD stage 4) renal failure, those treated in earlier stages had a substantially better renal survival.1,6,7
Early diagnosis is challenging for hematologists because up to 63%6 of patients with LCDD do not have a serum monoclonal whole immunoglobulin. A monoclonal protein in serum or urine is detected by electrophoresis/immunofixation in only ∼65% of cases,1,7 possibly because the κ FLC tends to aggregate and does not form a discrete, detectable electrophoretic band (see figure, panel B). On the other hand, the FLC ratio is abnormal in all patients with LCDD,1,6,7 suggesting that serum FLC measurement should be performed in the diagnostic workup of adults presenting with renal disease, independent of the presence of a serum or urine monoclonal protein. This may facilitate early diagnosis and prompt therapy. Diagnosis relies on renal biopsy and close collaboration between nephrologists and hematologists is essential.
Sayed and coworkers elected to use the FLC-based criteria for hematologic response validated in AL amyloidosis8 in their LCDD population, and they found that the outcome of patients who achieved VGPR or CR was characterized by an improvement of glomerular filtration rate in most patients, significantly diverging from the progressive decrease in renal function observed in subjects obtaining less than VGPR (see figure, panel A), thus validating the use of the hematologic response criteria developed for AL amyloidosis also in LCDD. Although the number of patients is small, a biomarker-based assessment of response of organs other than the kidney, analogous to that used in AL amyloidosis, seems applicable also in LCDD. For instance, the 2 patients with heart involvement who improved had a pronounced decrease in NT-proBNP, which is recognized as an indicator of cardiac response in AL amyloidosis.8,9
Although this study was not powered for comparison of treatment regimens, it confirms several previous observations that high-dose melphalan followed by autologous stem cell transplantation (HDM/ASCT) is well tolerated (also in patients with advanced kidney damage) and very effective. It also highlights that bortezomib treatment is indeed a targeted treatment of LCDD, providing rapid, profound (complete response in 8 of 9 patients) and sustained hematologic responses that translate into prolonged renal survival (as demonstrated by the need for dialysis). Similar findings have been reported by French investigators,7 who also showed that hematologic response rates were similar after HDM/ASCT and bortezomib-based regimens. These findings suggest that the latter can be considered as front-line therapy, particularly in patients with severe renal failure.
This is a new era for diseases caused by monoclonal LC deposition/aggregation; we now have effective therapies that can completely change the natural history of these conditions, making more acute the need for early diagnosis through improved awareness and wider use of the FLC assay in adults presenting with renal disease.
Conflict-of-interest disclosure: G.M. received honoraria from Millennium Takeda. G.P. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal