In this issue of Blood, Forero-Torres et al1 report encouraging results from a phase 2 study of brentuximab vedotin in the first-line treatment of older patients with Hodgkin lymphoma (HL). This is a population in whom frailty and comorbidity are common and outcomes poor when compared with those seen in younger patients.2 These features are closely linked. Doxorubicin-containing chemotherapy regimens such as doxorubicin, bleomycin, vinblastine, and dacarbazine or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone are considered inappropriate because of the presence of significant cardiac disease, other comorbidity(ies), or poor performance status, and, even if used, these regimens are frequently subject to dose reductions and treatment delays because of toxicity. As a consequence, either standard-of-care first-line regimens are not given at all or their optimal use is compromised. Alternative, less effective chemotherapy regimens or palliative radiotherapy approaches have until now been the only other options.
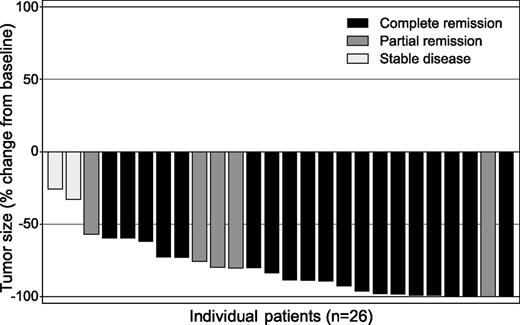
Tumor size reduction (measured by computed tomography scan) and overall response (measured by positron emission tomography scan) for each of 26 evaluable patients treated with brentuximab vedotin. Nineteen patients (73%) achieved complete response. See Figure 1 in the article by Forero-Torres et al that begins on page 2798.
Tumor size reduction (measured by computed tomography scan) and overall response (measured by positron emission tomography scan) for each of 26 evaluable patients treated with brentuximab vedotin. Nineteen patients (73%) achieved complete response. See Figure 1 in the article by Forero-Torres et al that begins on page 2798.
In their study, Forero-Torres et al treated 27 patients with a new diagnosis of classical HL. All were aged 60 or older, with a median age of 78 years, and 5 patients were aged 85 or older. Fifty-two percent were deemed ineligible for treatment with conventional multiagent chemotherapy due mainly to heart disease comorbidity, 67% of patients reported being “limited a lot” for at least one physical activity, and 30% had fallen at least once in the previous 6 months. Hence, there is no doubt that this was a frail, comorbid, and vulnerable group of patients.
Overall response rate to brentuximab vedotin, an antibody-drug conjugate targeting CD30,3 was 92% among the 26 efficacy-evaluable patients, with 19 patients (73%) achieving complete remission, 5 (19%) achieving partial remission, and 2 patients having stable disease (see figure). With a median observation time of 17 months, median progression-free survival was 10.5 months for all patients and 11.8 months for those achieving complete remission. Six patients have remained alive and progression-free for >12 months, with a median duration of time off treatment of 6.5 months. Overall survival ranged from 4.6 to 24.9 months with the median not yet reached.
Patients received a median of 8 cycles of treatment, with 8 patients completing 16 cycles. In general, brentuximab vedotin was well-tolerated and no infusion-related or hypersensitivity reactions were reported. All patients reported at least one adverse event, with peripheral neuropathy (21 patients, 78%), fatigue (12 patients, 44%), and nausea (12 patients, 44%) being the most common. Although these events were typically grade 1 or 2, 8 patients (30%) developed grade 3 neuropathy, most usually in the presence of coexisting diabetes mellitus or hypothyroidism. Myelosuppression was minimal and there was no thrombocytopenia or febrile neutropenia reported.
In terms of disease control rates and toxicity, these are very promising results, but the small number of patients treated mandates confirmatory studies. The investigators should be congratulated, however, for considering the needs of a group of patients poorly served by conventional treatments for HL who have been under-represented or absent from large randomized trials of therapy. They have shown that brentuximab vedotin, which already has an established role in the relapsed/refractory setting,4 is an effective single agent in the first-line treatment of older patients with classical HL and an extremely welcome new option for these individuals. It is encouraging that myelosuppression was not a significant toxicity in this study. However, physicians need to be aware of the risk of peripheral neuropathy, especially in patients with coexisting diabetes and hypothyroidism, and be ready to reduce and/or delay treatment for moderate toxicity and discontinue use if the symptoms become severe.
The challenge now is to determine whether these results, particularly in terms of duration of response, can be improved by combining brentuximab vedotin with other agents without adversely affecting patient tolerability. Good candidates for this role are the noncardiotoxic bendamustine—which has already been used in combination to very good effect in relapsed/refractory patients5 —and dacarbazine. These doublet drug approaches are currently under evaluation. Results will be awaited with interest, but the outcome of this study of brentuximab vedotin monotherapy makes clear that important progress has already been made in the treatment of older patients with HL, for whom therapeutic options have historically been extremely limited.
Conflicts-of-interest disclosure: The author has been compensated for taking part in advisory boards and speaking engagements for Takeda, Seattle Genetics, Cell Medica, and Novartis.