Key Points
CRP enhances antibody-mediated lung damage when infused into TRALI-resistant mice.
CRP and TRALI-inducing antibodies generate a synergistic increase in MIP-2 production and pulmonary neutrophil accumulation in vivo.
Abstract
Transfusion-related acute lung injury (TRALI) is a syndrome of respiratory distress triggered by blood transfusions and is the leading cause of transfusion-related mortality. TRALI has primarily been attributed to passive infusion of HLA and/or human neutrophil antigen antibodies present in transfused blood products, and predisposing factors such as inflammation are known to be important for TRALI initiation. Because the acute-phase protein C-reactive protein (CRP) is highly upregulated during infections and inflammation and can also enhance antibody-mediated responses such as in vitro phagocytosis, respiratory burst, and in vivo thrombocytopenia, we investigated whether CRP affects murine antibody–mediated TRALI induced by the anti–major histocompatibility complex antibody 34-1-2s. We found that BALB/c mice injected with 34-1-2s or CRP alone were resistant to TRALI, however mice injected with 34-1-2s together with CRP had significantly enhanced lung damage and pulmonary edema. Mechanistically, 34-1-2s injection with CRP resulted in a significant synergistic increase in plasma levels of the neutrophil chemoattractant macrophage inflammatory protein-2 (MIP-2) and pulmonary neutrophil accumulation. Importantly, murine MIP-2 is the functional homolog of human interleukin-8, a known risk factor for human TRALI. These results suggest that elevated in vivo CRP levels, like those observed during infections, may significantly predispose recipients to antibody-mediated TRALI reactions and support the notion that modulating CRP levels is an effective therapeutic strategy to reduce TRALI severity.
Introduction
Transfusion-related acute lung injury (TRALI) is characterized by respiratory distress following blood transfusions and is the leading cause of transfusion-related mortality.1,2 Approximately 80% of TRALI cases are associated with HLA- or human neutrophil antigen–specific antibodies present in donor blood.3,4 A 2-hit model was hypothesized to underlie antibody-mediated TRALI where the first hit comprises patient predisposing factors, such as inflammation, and the second hit is due to antibodies in the transfused blood.1 The pathogenesis of antibody-mediated TRALI, however, is still poorly understood, and controversy has arisen using animal models. For example, several cell types have been implicated in inducing lung damage by the TRALI-inducing anti–major histocompatibility complex (MHC) class I antibody 34-1-2s.5 Neutrophils were originally thought to be stimulated by 34-1-2s to produce reactive oxygen species that damaged the pulmonary endothelium in an Fc-dependent manner.1,5-13 Strait et al,14 however, showed that neutrophil involvement was limited and that activated endothelial cells and complement interactions were the primary events in TRALI induction. Furthermore, we demonstrated that TRALI induction was due to 34-1-2s binding to its cognate MHC class I antigens on monocytes, activating them to secrete macrophage inflammatory protein-2 (MIP-2), the murine equivalent of human interleukin-8 (IL-8), which was associated with pulmonary neutrophil recruitment.15 This event initiated subsequent lung damage in an Fc-dependent process.15
In the original study, TRALI induction in BALB/c mice injected with 34-1-2s was robust and significant lung damage was observed.5 Subsequently, however, TRALI-induction by 34-1-2s was lost and only BALB/c mice administered with the bacterial endotoxin lipopolysaccharide (LPS) succumbed to TRALI.16 The loss was associated with changes in animal housing that suggested that gut flora may be a predisposing factor. Our previous results also show that BALB/c mice are resistant to antibody-mediated TRALI induction but mice with severe combined immunodeficiency, lacking T cells, are hypersensitive to 34-1-2s.17
Recipient inflammation is a significant risk factor for TRALI induction. For example, in mice, LPS/platelet-induced neutrophil extracellular traps can be formed that cause lung damage and aspirin was found to attenuate the TRALI.18 In humans, a study showed that cardiac surgery patients have systemic inflammation consisting of increased plasma levels of IL-6 and IL-8, but plasma IL-6 and IL-8 levels were significantly higher in patients in whom TRALI developed than in controls.19 Others have also found that systemic inflammation associated with higher IL-8 levels before transfusion may be a significant risk factor for TRALI induction.20,21 In humans, severe inflammatory conditions can cause acute lung injury (ALI) without transfusion, such as sepsis, pneumonia, aspiration, multiple fractures, and pancreatitis.22,23 When such patients are transfused and ALI develops, the underlying major ALI risk factors appear to cause the ALI, and transfusion contributes little or nothing to the ALI.23
CRP is a well-established acute-phase protein and is used in clinical practice as a sensitive biomarker for infection and inflammation. CRP is produced by hepatocytes in response to inflammatory cytokines, in particular IL-6. CRP binds to cell wall polysaccharides of Streptococcus pneumonia24 or, more specifically, to phosphorylcholine residues. Phosphorylcholine is a component of cellular membranes and becomes exposed upon activation, such as upon damage,25 apoptosis,26 or oxidation.27 Recently, upon platelet oxidation, CRP was able to bind to platelet-phosphorylcholine and subsequently enhanced antibody-mediated respiratory burst and phagocytosis as well as antibody-mediated platelet destruction in vivo in an Fc-receptor–dependent manner.27 Although the latter occurred independently of complement,27 CRP has been known to be involved in activation of complement, particularly through interaction with C1q.28 In addition, it is known that CRP can also mediate a direct proinflammatory effect on endothelial cells.29 Considering that TRALI is primarily antibody mediated and that inflammation is clearly associated with TRALI in both animal and human studies,18-21 we investigated whether CRP could affect antibody-mediated TRALI induction in TRALI-resistant BALB/c mice. We report here that CRP can synergistically enhance 34-1-2s–induced TRALI induction in BALB/c mice in vivo, suggesting that this acute-phase protein could be a novel therapeutic target to reduce TRALI reactions.
Methods
Mice
Male BALB/c (H-2d, BALB/cAnNCrl) mice were obtained from Charles River Laboratories (Montreal, QC, Canada) and were housed for at least 1 week in our animal facility before initiation of experiments at an age of 8-10 weeks. All animal studies were approved by the St. Michael’s Hospital Animal Care Committee.
CRP and antibodies for injection into mice
CRP purified from human plasma (100% purity by sodium dodecyl sulfate electrophoresis) was purchased from Sigma-Aldrich (St. Louis, MO; cat# C4063). The mouse monoclonal antibody 34-1-2s (immunoglobulin G2a [IgG2a]) reacts against murine H-2Kd and H-2Dd MHC class I molecules and was purchased from Bio X Cell (West Lebanon, NH). The monoclonal antibody recognizes a monomorphic region on the MHC class I α-3 domain (independently of MHC/β2 microglobulin association) and also cross-reacts with MHC class I molecules from the b, q, s, and r strains of inbred mice. Isotype control mouse IgG2a was purchased from Bio X Cell.
Antibody-mediated BALB/c TRALI model
Mice were weighed and administered 34-1-2s or isotype mouse IgG2a antibody (4.5 mg/kg) with or without CRP (10 mg/kg) by a single IV (100 µL) tail vein injection. Ninety minutes after injection, mice were anesthetized with Avertin (2% final in phosphate-buffered saline [PBS], intraperitoneally), and blood was collected through cardiac puncture for plasma MIP-2 analysis and blood cell count determination. The lungs were harvested for determination of lung wet-to-dry (W/D) weight ratios and pulmonary neutrophil accumulation as well as for lung histology analysis.
Lung W/D weight ratio
Lung W/D weight ratios were determined as a measure of pulmonary edema. The right lung of each mouse was removed, weighed to determine the wet weight, dried in an oven at 60°C for 48 hours, and reweighed to obtain the dry weight. The W/D ratio was subsequently calculated as the net wet weight divided by the net dry weight.
Blood collection, plasma preparation, MIP-2 detection, and blood count measurements
Blood was collected through cardiac puncture into a 100-µL PBS/citrate phosphate dextrose adenine solution and plasma was subsequently obtained after two centrifugation steps of 2500g for 15 minutes. Plasma samples were stored at −80°C and analyzed in batch to determine the plasma MIP-2 levels. An MIP-2 enzyme-linked immunosorbent assay (ELISA) was performed using a solid-phase ELISA kit (mouse CXCL2/MIP-2 Quantikine ELISA Kit; R&D Systems, Minneapolis, MN), according to the manufacturer’s instruction, with an MIP-2 sensitivity of >1.5 pg/mL. For blood counts, collected whole blood was diluted 1/1000 in PBS/citrate phosphate dextrose adenine solution and immediately counted using a Coulter LH 780 Hematology Analyzer (Beckman Coulter, Mississauga, ON, Canada).
Pulmonary neutrophil accumulation
The chest cavity was exposed and the left lung was removed, homogenized, and filtered through a 40-µm cell strainer (BD Biosciences, Bedford, MA) with PBS. Red blood cells were lysed through addition of ammonium chloride/potassium bicarbonate lysis solution (0.15 M NH4Cl, 10 mM KHCO3, Na2EDTA [pH 7.2-7.4]) for 5 minutes on ice, and cells were eventually mounted on microscope slides by using a Shandon Cytospin 4 (ThermoFisher Scientific, Nepean, ON, Canada) and stained with a hematoxylin and eosin kit (Harleco-Hemacolor; EMD Chemicals, Darmstadt, Germany). The slides were then examined under Permount (Fisher Scientific Company, Ottawa, ON, Canada) using a Nikon Eclipse E800 microscope equipped with a ×40/0.75 objective lens. Polymorphonuclear cells were determined by counting the total number of nucleated cells in four randomly selected and nonoverlapping fields, and neutrophil numbers were enumerated by using ImageJ software. The pulmonary neutrophil percentage was calculated by the total number of neutrophils divided by total number of nucleated cells × 100. Averages of 4 fields were taken.
Lung tissue histology
For lung tissue histology, a segment of the left lung was removed and fixed in 10% formalin, paraffin embedded, and sectioned with a microtome. The sections were subsequently stained with hematoxylin and eosin using a Leica AutoStainerXL (Leica Biosystems, Nussloch, Germany). The slides were examined under Permount (Fisher Scientific Company, Ottawa, ON, Canada) using a Nikon Eclipse E800 microscope equipped with a ×20 and ×40/0.75 objective lens.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.07 software for Windows (GraphPad Software, San Diego, CA) with statistical significance set at P < .05.
Results
BALB/c mice are resistant to antibody-mediated TRALI induction but undergo antibody-mediated TRALI upon injection of CRP
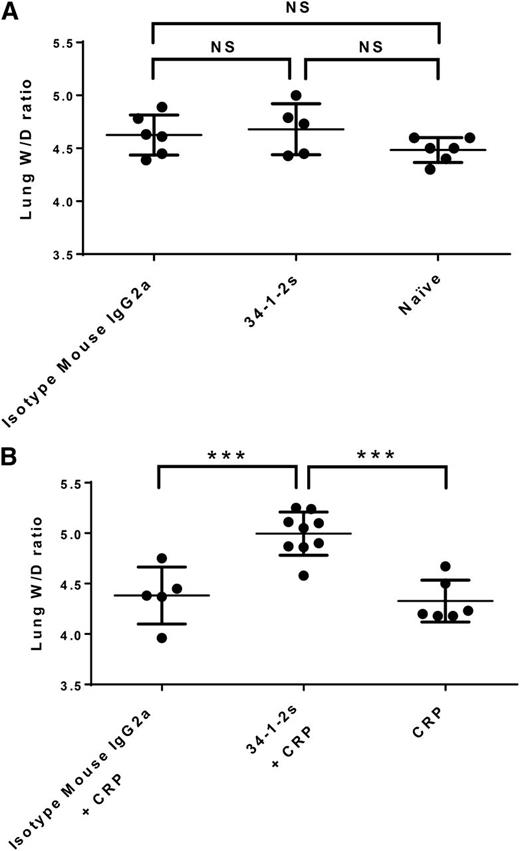
As observed previously,17 injection of 34-1-2s alone did not result in lung damage (pulmonary edema) in BALB/c mice, as lung W/D weight ratios were similar to control BALB/c mice that received isotype mouse IgG2a antibody alone or naïve mice (Figure 1A). Injection of 34-1-2s together with CRP, however, resulted in significantly higher lung W/D weight ratios compared with mice injected with either isotype IgG2a antibody together with CRP or with CRP alone, with at least 55% of the mice in the CRP + 34-1-2s group displaying a lung W/D weight ratio of higher than 5, which is considered to represent severe lung damage (Figure 1B).
Lung W/D weight ratios in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. (A) Injection of isotype mouse IgG2a antibody or 34-1-2s alone did not result in lung damage, similar to untreated naïve mice. (B) Injection of CRP together with 34-1-2s results in increased lung W/D weight ratios compared with CRP injection with isotype IgG2a or CRP alone. Ninety minutes after antibody and/or CRP injections, mice were euthanized and lungs were harvested. Each dot represents 1 mouse, and error bars represent standard deviation (SD). Only comparisons of significance are shown and determined by 1-way analysis of variance (ANOVA) with Tukey’s post hoc. NS, nonsignificant. ***P < .001.
Lung W/D weight ratios in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. (A) Injection of isotype mouse IgG2a antibody or 34-1-2s alone did not result in lung damage, similar to untreated naïve mice. (B) Injection of CRP together with 34-1-2s results in increased lung W/D weight ratios compared with CRP injection with isotype IgG2a or CRP alone. Ninety minutes after antibody and/or CRP injections, mice were euthanized and lungs were harvested. Each dot represents 1 mouse, and error bars represent standard deviation (SD). Only comparisons of significance are shown and determined by 1-way analysis of variance (ANOVA) with Tukey’s post hoc. NS, nonsignificant. ***P < .001.
Plasma MIP-2 levels and pulmonary neutrophil accumulation are synergistically increased by injection of antibody and CRP during antibody-mediated TRALI
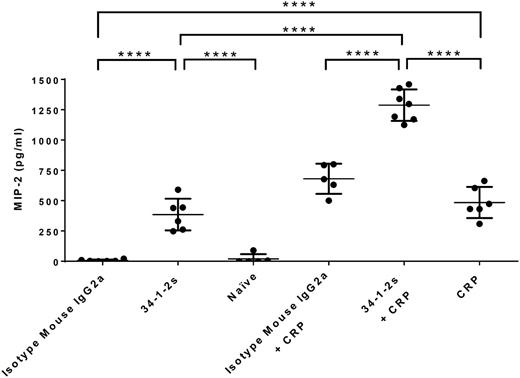
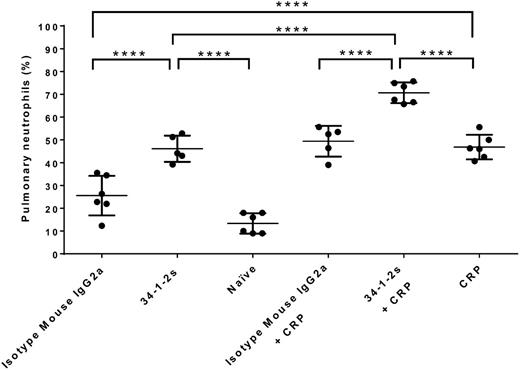
Plasma MIP-2, the murine equivalent of human IL-8, was measured by ELISA. Compared with control naïve and isotype mouse IgG2a-treated mice, 34-1-2s and CRP injection alone resulted in significant plasma MIP-2 production (385.5 ± 130.7 pg/mL and 485.1 ± 128.6 pg/mL, respectively; Figure 2). In contrast, however, when 34-1-2s was coinjected with CRP plasma, MIP-2 levels were synergistically increased (1288 ± 129.8 pg/mL; Figure 2). These MIP-2 values were associated with relatively similar increased pulmonary neutrophil percentages, as both 34-1-2s and CRP alone were able to recruit neutrophils to the lung (46.1% ± 5.76% and 46.85% ± 5.38%, respectively; Figure 3), whereas 34-1-2s injected together with CRP resulted in significantly enhanced pulmonary neutrophil accumulation (70.67% ± 4.51%; Figure 3).
Plasma MIP-2 levels in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection and blood was collected, followed by plasma MIP-2 level determination. Each dot represents 1 mouse, and error bars represent SD. Only comparisons of interest are shown and determined by 1-way ANOVA with Tukey’s post hoc. ****P < .0001.
Plasma MIP-2 levels in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection and blood was collected, followed by plasma MIP-2 level determination. Each dot represents 1 mouse, and error bars represent SD. Only comparisons of interest are shown and determined by 1-way ANOVA with Tukey’s post hoc. ****P < .0001.
Pulmonary neutrophil percentages in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection, and lung harvesting was performed. Each dot represents 1 mouse, and error bars represent SD. Only comparisons of interest are shown and determined by 1-way ANOVA with Tukey’s post hoc. ****P < .0001.
Pulmonary neutrophil percentages in BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection, and lung harvesting was performed. Each dot represents 1 mouse, and error bars represent SD. Only comparisons of interest are shown and determined by 1-way ANOVA with Tukey’s post hoc. ****P < .0001.
CRP enhancement of antibody-mediated TRALI results in severe lung damage as evidenced by histological analysis
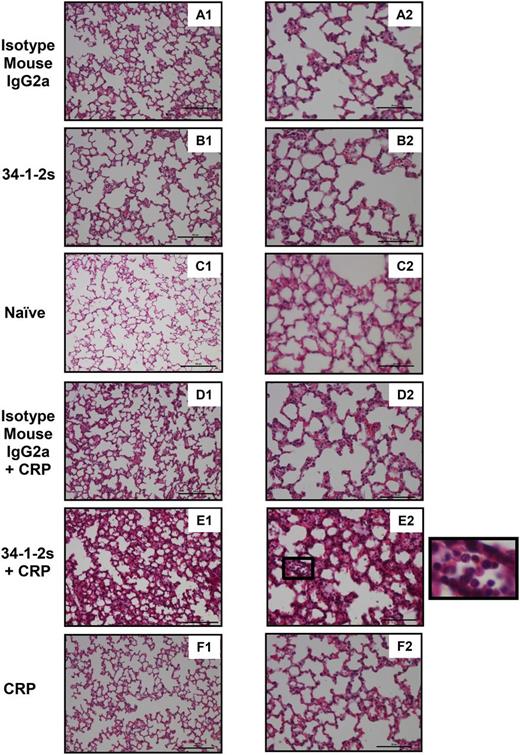
In order to directly assess the lung damage that occurred upon coinjection of 34-1-2s together with CRP, lung tissue histology was performed. Compared with all the other mouse groups (Figure 4A-D,F), only the lungs from mice treated with both 34-1-2s and CRP showed significant lung damage characterized by increased alveolar septal thickening, proteinaceous debris filling the airspaces, and neutrophil infiltration in the alveolar space (Figure 4E).
Lung tissue histology from BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection and lungs were harvested for sectioning and histology analysis. Panels A1 to F1 and A2 to F2 represent lung tissue images taken at original magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in E2 (lung section from mice treated with 34-1-2s + CRP) is depicted alongside and shows alveolar neutrophil infiltration. Scale bars represent 100 µM in panels A1 to F1 and 50 µM in A2 to F2.
Lung tissue histology from BALB/c mice receiving the indicated transfusions of antibody and/or CRP. Mice were euthanized after 90 minutes following antibody and/or CRP injection and lungs were harvested for sectioning and histology analysis. Panels A1 to F1 and A2 to F2 represent lung tissue images taken at original magnification ×20 and ×40, respectively. Representative images of each indicated group are shown. A zoom of indicated square in E2 (lung section from mice treated with 34-1-2s + CRP) is depicted alongside and shows alveolar neutrophil infiltration. Scale bars represent 100 µM in panels A1 to F1 and 50 µM in A2 to F2.
CRP enhancement of antibody-mediated TRALI results in a significant and isolated thrombocytopenia
Blood cell counts were measured, and it was observed that compared with the naïve mice (889.40 ± 116.70 × 109 platelets/L; Table 1), those injected with both 34-1-2s and CRP had a significant and isolated thrombocytopenia (544.40 ± 107.30 × 109 platelets/L; Table 1).
Blood cell counts of BALB/c mice 90 minutes posttransfusion of 34-1-2s and CRP, compared with naïve control mice
| Blood cell type . | Naïve (control) . | 34-1-2s + CRP . | Statistical significance . |
|---|---|---|---|
| White blood cells (109/L) | 5.11 ± 1.47 | 6.28 ± 0.57 | NS |
| Red blood cells (1012/L) | 10.06 ± 0.44 | 10.85 ± 0.84 | NS |
| Platelets (109/L) | 889.40 ± 116.70 | 544.40 ± 107.30 | P < .001 |
| Blood cell type . | Naïve (control) . | 34-1-2s + CRP . | Statistical significance . |
|---|---|---|---|
| White blood cells (109/L) | 5.11 ± 1.47 | 6.28 ± 0.57 | NS |
| Red blood cells (1012/L) | 10.06 ± 0.44 | 10.85 ± 0.84 | NS |
| Platelets (109/L) | 889.40 ± 116.70 | 544.40 ± 107.30 | P < .001 |
Data represent average counts ± SD based on 6 BALB/c mice per group. Statistical comparisons were made using a 2-tailed unpaired Student t test. NS, nonsignificant.
Discussion
In the current study, we propose a novel antibody-mediated TRALI mechanism in the setting of inflammation, where CRP synergistically increases the ability of 34-1-2s to mediate TRALI induction via enhanced stimulation of MIP-2 secretion and increased pulmonary neutrophil accumulation.
Previously, LPS was shown to enable 34-1-2s–mediated TRALI,16 but in the current study, we focused fully on CRP, which, in contrast to LPS, is a general biomarker for inflammation and acute infections, including both gram-positive and gram-negative bacterial infections.
Severe lung damage was observed upon injection of CRP together with 34-1-2s, as evidenced by increased lung W/D ratios of higher than 5, in the majority of the cases. Moreover, the severity of the lung damage was underscored by histological analysis, showing increased alveolar septal thickening, proteinaceous debris filling the airspaces, and neutrophil infiltration in the alveolar space, all signs of severe acute lung injury.30 These increased lung W/D ratios were accompanied by a synergistic increase in MIP-2 levels and pulmonary neutrophil numbers. MIP-2 is a potent neutrophil chemokine and is the murine functional homolog of human IL-8. It shares the same extracellular loop reactive+ motif (Glu-Leu-Arg) for the chemoattractant properties of IL-8.31,32 We previously found that MIP-2 plays a central role in 34-1-2s–mediated TRALI-induction severe combined immunodeficiency mice,15 and because IL-8 is associated with an increased risk in human TRALI,20,21 we measured MIP-2 values in our BALB/c TRALI model. The current findings show that both CRP and 34-1-2s alone induce MIP-2 production in vivo, although neither is sufficient to induce TRALI. The source of MIP-2 in our study is unknown, but our previous work suggests that the primary source of the chemokine is monocytes,15 and, of interest, others have shown that CRP can augment the secretion of IL-8 by human peripheral blood monocytes.33 When CRP and 34-1-2s were combined, however, significant lung damage occurred, and this is associated, mechanistically, with significant levels of MIP-2 resulting in an increased pulmonary neutrophil recruitment. Both 34-1-2s and CRP separately have been shown to alter endothelial cells,14,29 and it may be that together, they directly and synergistically potentiate damage to the pulmonary endothelium and increase pulmonary leakage. We are currently studying this.
Upon infusion of 34-1-2s and CRP together, we observed a significant decrease in platelet counts compared with naïve control mice, and we did not observe any differences regarding white blood cell or red blood cell counts. Although transient leukopenia has been observed in human TRALI patients,34-37 it has often been an infrequent finding, most likely due to the dynamic nature of the leukopenia.34 Thrombocytopenia, on the other hand, has also been reported in human TRALI patients but appears to be more durable than the leukopenia.34-37 Therefore, these laboratory parameters in human TRALI patients seem to be in accordance with our animal model of TRALI. The decreased platelet counts we observed may be explained by a recent study demonstrating that CRP together with antiplatelet antibodies can enhance platelet breakdown through Fc-receptor–mediated phagocytosis,27 considering that 34-1-2s is also able to bind to platelets. Importantly, our MIP-2 and blood cell count measurements appear to be in accordance with human TRALI, underlining the clinical relevance of our TRALI animal model.
The current study supports a role for CRP as a novel factor involved in the first hit of TRALI, as it is upregulated during inflammation. On the other hand, as CRP exerts its actual effects together with the antibody, the mode of action of CRP could be seen as a simultaneous first as well as second hit. CRP plays a major role in MIP-2 (IL-8) secretion but may also be involved in other inflammatory processes, as CRP has been able to stimulate antibody-mediated reactive oxygen species production by neutrophils27 and has also been able to bind to endothelium29 and interact with complement.28 Further studies should clarify the extent of CRP’s involvement in antibody-mediated TRALI.
Regarding the known role of CRP in general inflammation and the increased risk of TRALI during inflammation, the current study exposes a novel link between CRP and TRALI induction. CRP was able to enhance in vivo MIP-2 secretion on its own but together with 34-1-2s synergistically increased the chemokine’s secretion, pulmonary neutrophil recruitment, and lung damage. Therefore, CRP targeting may prove to be a therapeutic approach in reducing antibody-mediated TRALI severity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Health Canada and Canadian Blood Services (J.W.S.). R.K. is the recipient of a postdoctoral fellowship from Canadian Blood Services. S.S. is the recipient of a Canadian Blood Services Summer Studentship.
Authorship
Contribution: R.K. designed research, performed all experiments, collected data, analyzed and interpreted data, performed statistical analyses, made the figures, and wrote the paper; M.K., S.S., J.L., and Y.L. performed experiments and collected and interpreted data; and J.W.S. provided financial resources, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St. Michael’s Hospital, 30 Bond St, Toronto, ON, Canada M5B 1W8; e-mail: semplej@smh.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal