In this issue of Blood, Kapur et al identify the acute-phase protein C-reactive protein (CRP), a general biomarker for infection and inflammation, as a potent mediator of antibody-dependent transfusion-related acute lung injury (TRALI). This study provides a novel and direct link between inflammation and TRALI, opening doors for possible new approaches in targeting TRALI.1
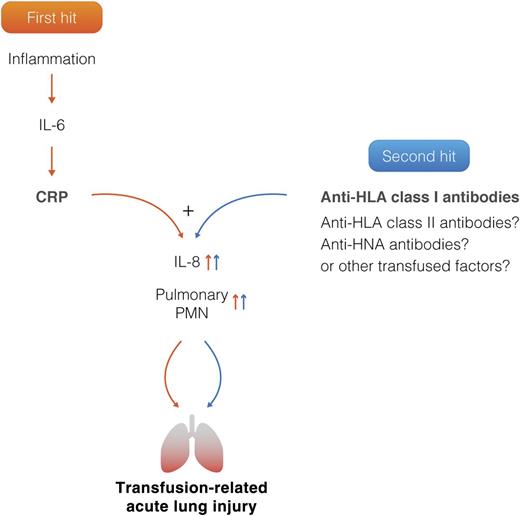
Two-hit model of TRALI. The first hit represents patient predisposing factors including CRP, which is now identified as a novel first hit by the current study. The second hit represents antibodies and factors directly related to the transfused blood product. Inflammation causes an increase in IL-6, which stimulates a rapid elevation of CRP. CRP together with anti-HLA class I antibodies trigger a synergistic increase in both IL-8 and pulmonary polymorphnuclear neutrophil (PMN) accumulation, resulting in TRALI induction. CRP or anti-HLA class I antibodies alone are not sufficient to induce TRALI. Professional illustration by Somersault18:24.
Two-hit model of TRALI. The first hit represents patient predisposing factors including CRP, which is now identified as a novel first hit by the current study. The second hit represents antibodies and factors directly related to the transfused blood product. Inflammation causes an increase in IL-6, which stimulates a rapid elevation of CRP. CRP together with anti-HLA class I antibodies trigger a synergistic increase in both IL-8 and pulmonary polymorphnuclear neutrophil (PMN) accumulation, resulting in TRALI induction. CRP or anti-HLA class I antibodies alone are not sufficient to induce TRALI. Professional illustration by Somersault18:24.
TRALI is a syndrome characterized by acute respiratory distress resulting in acute lung injury within 6 hours upon blood transfusion. In the majority of the cases, antibodies against HLAs and/or human neutrophil antigen (HNA) present in the transfused product are thought to be responsible for initiating TRALI.2 The implementation of TRALI mitigating strategies, such as the use of male-only transfused blood products, has decreased the incidence of antibody-mediated TRALI, however, TRALI still remains the leading cause of transfusion-related mortality and is thus an important clinical problem.3 Generally, TRALI is assumed to result from 2 hits, the first hit being caused by the underlying clinical condition of the patient, whereas the second hit occurs when the antibodies or factors are transferred to the recipient during the transfusion.2 The pathogenesis is still poorly understood but several cellular processes have been described such as neutrophil activation, pulmonary endothelial barrier function disruption, and the involvement of monocytes.4-6 In addition, several TRALI risk factors have been described in recipients which include increased levels of interleukin-8 (IL-8), liver surgery, chronic alcohol abuse, shock, higher peak airway pressure while being mechanically ventilated, smoking, and positive intravascular fluid balance.7 In a prepost study, IL-6 and IL-8 were found to be elevated before and after transfusion in patients with TRALI.8 These studies clearly identify systemic inflammation as a major risk factor for developing TRALI.
In the current study, Kapur et al provide further support for inflammation as a prerequisite for antibody-mediated TRALI and offer novel insights into TRALI pathogenesis by linking CRP to increased risk of antibody-mediated TRALI induction.1 CRP is an acute-phase protein, produced in the liver, and is clinically regarded as a general biomarker for acute inflammation and infections. As CRP levels increase rapidly during inflammation, and because inflammation is a risk factor for TRALI, the role of CRP in antibody-mediated TRALI was investigated. The authors used a BALB/c mouse model for TRALI which is based on injection of the anti–major histocompatibility complex (MHC) class I antibody, 34-1-2s; BALB/c mice are known to be resistant to TRALI, unless primed with the gram-negative bacterial endotoxin lipopolysaccharide (LPS). The authors did not perform a direct comparison of the effects of LPS vs CRP, but focused the studies solely on CRP. They elegantly demonstrate that infusion of purified CRP preparation in combination with 34-1-2s significantly enhances TRALI induction in the normally TRALI-resistant mice, as measured by increased pulmonary edema which was further underscored by lung histology displaying signs of severe acute lung damage. CRP or 34-1-2s injection alone was insufficient to cause the lung damage but did result in increased macrophage inflammatory protein-2 (MIP-2) levels, the murine functional homolog of human IL-8 and increased pulmonary neutrophil recruitment. CRP combined together with 34-1-2s, however, resulted in a synergistic increase in both MIP-2 secretion as well as in increased pulmonary neutrophil numbers that associated with the severe lung damage (see figure). In addition, they demonstrate that infusion of CRP together with 34-1-2s results in an isolated thrombocytopenia without leukopenia, a finding which is also observed in human TRALI, and most likely reflects the dynamic and transient nature of the leukopenia vs the more durable nature of the thrombocytopenia.
The key finding of current study is that CRP plays a major role in TRALI pathogenesis by synergizing with MHC class I antibodies to increase plasma MIP-2 (IL-8) levels and pulmonary neutrophil recruitment and subsequent lung injury. The study does not exclude the possibility that CRP may also be involved in other inflammatory processes leading to TRALI, including enhancing antibody-mediated respiratory burst by neutrophils,9 synergizing with MHC class I antibodies in binding to the endothelium,5,10 and causing pulmonary vascular leakage and/or complement cascade activation. The source of the MIP-2 secretion was not shown; however, their previous work demonstrated the source of MIP-2 secretion to be blood monocytes.9
In summary, this study provides compelling and encouraging evidence depicting CRP as a novel first-hit player in TRALI, and strengthens the involvement of systemic inflammation and IL-8 in TRALI induction. Follow-up work should expand on the role of CRP in other antibody and nonantibody TRALI and focus on investigating the extent of CRP’s involvement in TRALI, for instance, with regards to the production of reactive oxygen species, complement cascade stimulation, and disturbance of pulmonary vascular endothelium function. Furthermore, therapeutically, downmodulation of CRP levels could be an interesting strategy to explore in reducing the severity of antibody-mediated TRALI.
Conflict-of-interest disclosure: The author declares no competing financial interests.