In vitro studies strongly implicate the talin-binding Ras-related protein 1 (Rap1) effector, Rap1-guanosine triphosphate–interacting adapter molecule (RIAM), in integrin activation.1 Yet, the RIAM knockout mouse is viable and fertile and exhibits no platelet adhesion or aggregation defects,2 casting doubt on the in vivo role of RIAM. In this issue of Blood, Su et al3 and Klapproth et al4 now show that RIAM is required for β2 integrin–dependent leukocyte adhesion and trafficking in vitro and in vivo, but apparently not for all Rap- and talin-meditated activation of β1 and β3 integrins.
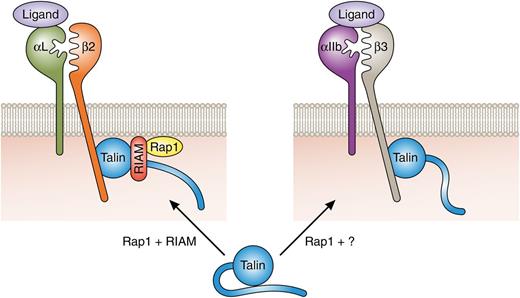
Rap1-mediated integrin activation. Talin-mediated activation of β2 integrins requires Rap1 and RIAM expression. A RIAM-Rap1 complex targets talin to the plasma membrane and contributes to conformational activation of talin, resulting in β2 integrin tail binding and integrin activation. Activation of platelet αIIbβ3 is talin and Rap1 dependent but independent of RIAM. The relevant Rap1 effector is yet to be identified. RIAM dependency of α4β1 activation is cell-type dependent (not shown). For clarity, additional proteins contributing to Rap-dependent and -independent integrin activation are not depicted. Professional illustration by Patrick Lane, ScEYEnce Studios.
Rap1-mediated integrin activation. Talin-mediated activation of β2 integrins requires Rap1 and RIAM expression. A RIAM-Rap1 complex targets talin to the plasma membrane and contributes to conformational activation of talin, resulting in β2 integrin tail binding and integrin activation. Activation of platelet αIIbβ3 is talin and Rap1 dependent but independent of RIAM. The relevant Rap1 effector is yet to be identified. RIAM dependency of α4β1 activation is cell-type dependent (not shown). For clarity, additional proteins contributing to Rap-dependent and -independent integrin activation are not depicted. Professional illustration by Patrick Lane, ScEYEnce Studios.
Talin binding to the cytoplasmic tails of the β subunits of integrin adhesion receptors is key for the activation of most integrin heterodimers.1 The molecular mechanisms by which talin binds integrin tails and triggers integrin activation are now well accepted,1 but how talin binding is regulated is less well understood. It is, however, generally agreed that talin must be recruited to the plasma membrane and an inhibitory talin head-rod interaction released to facilitate talin head binding to integrins and their subsequent activation.1 Although a number of mechanisms have been proposed,1 1 influential study, where agonist-induced activation of the platelet integrin αIIbβ3 was reconstructed in heterologous cells, highlighted roles for the Rap1 effector RIAM in talin-mediated integrin activation.5 These initial results, along with biochemical and cell biological follow-up work, led to a model where RIAM binds to sites in the talin rod and this RIAM-talin complex is recruited to activated membrane-bound Rap15-8 (see figure). Indeed, fusing the minimized talin-binding portion of RIAM to the Rap1A membrane-targeting sequence was sufficient to target talin to the plasma membrane and to activate integrins.6 More recently, a RIAM-binding site was also identified in the talin head and RIAM binding at this site was shown to contribute to release of inhibitory talin head-rod interactions.9 RIAM may, therefore, mediate both membrane recruitment and conformational activation of talin.
Loss of β1 integrin, talin-1, or both Rap1A and Rap1B produces embryonic lethality in mice,1,10,11 whereas mice or humans deficient in β3 integrins exhibit Glanzmann thrombasthenia phenotypes including defective platelet aggregation and prolonged bleeding.11 Therefore, given the extensive cellular and biochemical data implicating RIAM in talin-mediated β1 and β3 integrin activation, it came as a considerable surprise when earlier this year a RIAM knockout mouse was reported to be viable with no major developmental phenotype and strikingly no evident platelet defects.2 The articles by Klapproth et al4 and Su et al3 now report analyses of an independently generated RIAM knockout mouse. These new studies confirm that RIAM-deficient mice are viable and fertile,3,4 and that they lack platelet defects,3 but both also show that the absence of RIAM produces severe defects in β2 integrin–mediated leukocyte adhesion and trafficking. In fact, despite normal cell surface integrin levels, the RIAM-null phenotype resembles the leukocyte adhesion deficiency seen in β2 integrin null mice.11 T-lymphocyte–specific double knockout of Rap1A and Rap1B also produces an adhesion deficiency3 as does T-cell or hematopoietic-specific knockout of talin 1.4 These new data, therefore, support a key role for RIAM in Rap1- and talin-mediated β2 integrin activation. Notably, when compared quantitatively, loss of talin produced more severe adhesion phenotypes than loss of RIAM,4 presumably due to alternative pathways of talin activation. A more detailed side-by-side comparison of the Rap1-deficient and RIAM-deficient T cells may help determine whether RIAM mediates all Rap1-dependent effects on β2 integrin activation.
Strikingly, when bone marrow–derived neutrophil adhesion to the α4β1 ligand vascular cell adhesion molecule 1 (VCAM-1) was assessed, RIAM deficiency only partially inhibited adhesion whereas β2 integrin–mediated adhesion to intercellular cell adhesion molecule 1 (ICAM-1) or fibrinogen was strongly inhibited.4 Talin-1 knockout strongly inhibited adhesion on all 3 ligands. Assessing integrin activation state with conformation-specific antibodies supports the adhesion assay results. Thus, although both β1 and β2 integrin–mediated neutrophil adhesion is talin dependent, only β2 integrin adhesion is strongly RIAM dependent. Likewise, T-cell adhesion to VCAM-1 is less strongly inhibited by RIAM knockout than adhesion to ICAM-1, and B-cell adhesion to VCAM-1 is largely independent of RIAM.3 Finally, as previously reported,2 loss of RIAM had no effect on platelet function nor on activation of the platelet integrin αIIbβ3 as assessed using reporter antibodies.3 Thus, there is considerable integrin and cell-type specificity in the requirement of RIAM for integrin activation and, even when expressed in the same cell, β2 integrins are more dependent on RIAM than β1 integrins. This latter result, along with expression profiling, shows that the differential responses are not due to compensation for RIAM deficiency by expression of the related family member lamellipodin.
The work of Klapproth et al4 and Su et al3 highlights areas requiring further study to provide a complete understanding of control of integrin activation. Their data strongly suggest that RIAM-independent talin activation occurs in many or most adherent cell types (supporting normal development in the RIAM-null mouse) and in platelets. The existence of alternative talin activation methods was already appreciated1 ; indeed, vinculin competes with RIAM for binding to talin rod and is implicated as an alternative talin activator in mature adhesions.7 However, it is notable that Rap1-null platelets exhibit adhesion defects under conditions where none are evident in RIAM-deficient platelets.2,3,12 Another Rap1 effector, RAPL, has been implicated in integrin function but, like RIAM-null mice, RAPL-null mice exhibit leukocyte adhesion deficiencies and no platelet defects have been reported.13 This suggests that additional Rap-dependent talin activation pathways remain to be discovered. Furthermore, how RAPL- and RIAM-mediated signals are integrated in leukocytes is currently unclear. Another major question is the mechanism of specificity in integrin activation. Most integrins require talin but RIAM is selectively important for β2 integrins and to a lesser extent for β1 integrins. The basis for this is unknown although Klapproth et al4 suggest the possibility of differential integrin localization in specific membrane compartments. Altogether, these recent findings validate the in vivo role of Rap1–RIAM in talin-mediated β2 integrin activation. Moreover, they illustrate that although talin is a common final step in integrin activation, pathways upstream of talin appear to be integrin specific, allowing for the fine tissue-specific tuning of integrin-mediated adhesion.
Conflict-of-interest disclosure: The author declares no competing financial interests.