In this issue of Blood, O’Brien et al report mature results of initial treatment of chronic lymphocytic leukemia (CLL) with the PI3Kδ inhibitor idelalisib plus rituximab, and demonstrate that the combination is extremely effective with manageable toxicity in an older patient population.1
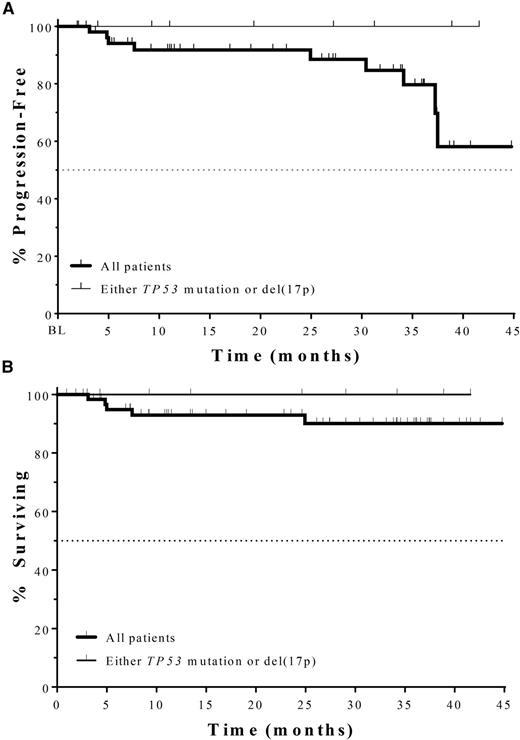
PFS (A) and overall survival (“Surviving”) (B) of older CLL patients who received idelalisib plus rituximab as initial therapy. No events have occurred among 9 TP53-mutated patients. See Figure 3 in the article by O’Brien et al which begins on 2686.
PFS (A) and overall survival (“Surviving”) (B) of older CLL patients who received idelalisib plus rituximab as initial therapy. No events have occurred among 9 TP53-mutated patients. See Figure 3 in the article by O’Brien et al which begins on 2686.
In the last several years, a revolution has occurred in the therapy of relapsed CLL. The approvals by the Food and Drug Administration of the Bruton's tyrosine kinase inhibitor ibrutinib for relapsed CLL patients and those with 17p deletion, as well as the approval of idelalisib with rituximab for relapsed CLL patients in whom rituximab is appropriate therapy, have led to the rapid adoption of these targeted therapies as the agents of choice for relapsed CLL. The effectiveness of these drugs, particularly in patients with high-risk cytogenetic abnormalities like 17p deletion, has naturally led to interest in their use for initial therapy, particularly in older patients in whom chemoimmunotherapy is less well-tolerated. Yet upfront data have been quite limited.
In this article, O’Brien et al present the first clinical trial of idelalisib with rituximab for the initial therapy of CLL in patients with a median age of 71 years, 42% of whom had advanced-stage disease. The overall response rate is 97%, with 19% being complete responders, none of whom have progressed to date. The progression-free survival (PFS) is an impressive 83% at 36 months, with only 4 events of disease progression, despite only 23 of 64 patients currently continuing on idelalisib (see figure). Among the highest-risk TP53-mutated patients (n = 9), the overall response rate is 100% and none have progressed, consistent with the known excellent activity of idelalisib in relapsed patients in this high-risk group.2 Although this was a phase 2 study, these results compare very favorably with the current standard of care for this patient population—obinutuzumab chlorambucil—which has a median PFS of 29.2 months in a phase 3 study,3 and are similar to the smaller phase 1b/2 study of ibrutinib, which showed a PFS of 96% at 30 months,4 with only 2 TP53-mutated patients. These results certainly justify registration trials of idelalisib in this upfront setting.
Toxicity was not insubstantial, however, with adverse events being the primary reason for discontinuation, occurring in 42% of patients. Grade 3 or higher diarrhea/colitis was seen in 42% of patients, compared with 14% in a recent summary of toxicity in 8 relapsed studies of idelalisib5 and 6% in the phase 1 study in very heavily pretreated patients. Grade 3 or greater transaminitis was seen in 23% of patients, compared with 14% in the early-relapse studies5 and 2% in the phase 1 study. Two cases of (fatal) pneumonitis and 2 cases of (reversible) pulmonary fibrosis were also observed.
This unique pattern of toxicity with idelalisib, namely colitis, hepatitis, and pneumonitis, is consistent across studies and is seen here to be paradoxically higher in untreated patients. This paradox could be resolved by an immunologic mechanism that might be more intact in less heavily treated patients. In this trial, these toxicities were primarily managed by holding drug, which is essential; but in addition, it has become increasingly clear that corticosteroids are a very effective treatment.6 Two recent studies characterizing the pathology of idelalisib-induced colitis have found CD8+ T-cell infiltrates associated with crypt apoptosis as a common feature.7,8 Interestingly, the target of idelalisib, namely the δ isoform of the PI3K p110 catalytic subunit, is known to be critical for the survival and function of regulatory T cells,9 and genetic mutations that disrupt regulatory T-cell function in mice and humans lead to a very similar autoimmune syndrome of hepatitis, enteritis, and pneumonitis.10 In a mouse model of inflammatory bowel disease, adoptive transfer of wild-type T-regulatory cells, but not regulatory T cells deficient in PI3Kδ, abolishes autoimmune colitis.9 Taken together, these findings suggest that idelalisib toxicities may be a result of on-target inhibition of p110 δ in regulatory T cells. Correlative studies in ongoing clinical trials will be needed to test this hypothesis in patients. Meanwhile, a high index of suspicion for autoimmune toxicity in patients on idelalisib, with early drug hold and consideration of corticosteroids once infection is ruled out or treated, is essential to optimize delivery of this highly effective therapy.
This study truly highlights both the remarkable efficacy and the typical toxicities of idelalisib. Of interest is the observation that 83% of patients were progression-free at 36 months, even though only 23 of 64 were still taking the drug (36%). In contrast to the current treatment paradigm, this finding suggests that many patients have durable remissions, even after stopping the drug, and raises the possibility of time-delimited therapy that might preserve benefit and limit toxicity. Alternatively, a better biological understanding of the likely immunologic toxicity may enable optimal patient selection and/or design of rational combinations that minimize toxicity. It is a brave new world in CLL therapy, with still much to learn to optimize our use of our incredibly effective new targeted therapies, so stay tuned for more rapid advances in the coming years!
Conflict-of-interest disclosure: The author has served as a consultant for Roche/Genentech, Pharmacyclics, Gilead, Infinity, and Janssen.