Abstract
Background: Recent clinical trials have demonstrated the efficacy and safety of gene therapy utilizing HIV-derived lentiviral vectors (LVs) for blood disorders. However, the LV requirements and clinical ex vivo cell transduction protocols used in these studies exposes the limitations of the technology and beckons the need for improved LV manufacturing and clinical transduction efficiency. Many methods have been devised to enhance efficiency, although none have circumvented the exorbitant amounts of virus required to achieve therapeutic HSC transduction. Furthermore, prolonged ex vivo cell culture is necessary to achieve sufficient transduction despite exposure to toxic byproducts of LV production. To that end, we developed a novel, scalable microfluidic for clinical LV transduction that leverages mass transfer principles to significantly reduce the amount of LV required to achieve therapeutic levels of gene transfer and transduction time by more efficiently exposing cells to virus.
Results: Jurkats were transduced with a GFP-encoding clinical LV in microfluidics with surface areas (SAs) comparable to the bottom surface of 96 and 6-well plates. Microfluidic transductions were compared to well plate transductions with matched SA, cell numbers, viral particles, and incubation times. After LV incubation, cells were removed from the microfluidics and well plates, spun down, re-suspended with fresh media, and cultured for at least 72 hours at 37°C and 5% CO2. Cells were assessed for GFP expression with flow cytometry. Preliminary mouse studies utilized Sca+ cells isolated from CD45.1 donor mice via positive selection. The cells were transduced in the scaled up microfluidic with a bioengineered coagulation factor VIII (fVIII) transgene encoding LV and transplanted into host hemophilia A mice after myeloablative conditioning. Two weeks post-transplantation, blood samples were taken from the recipient animals and assayed for donor cell engraftment by flow cytometry and plasma fVIII activity by chromogenic assay.
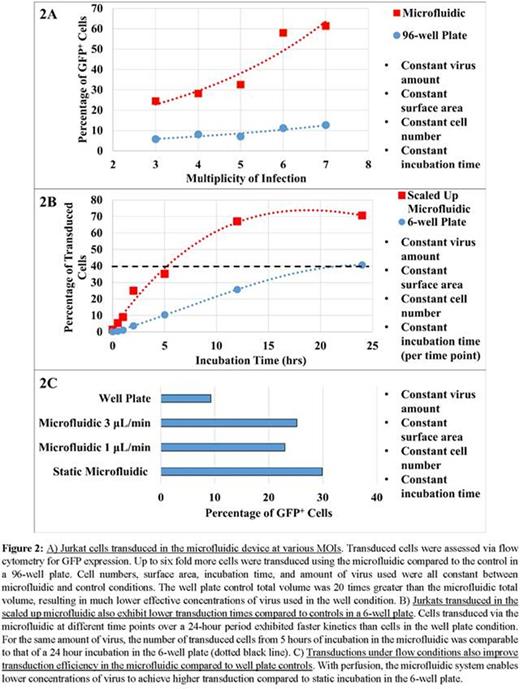
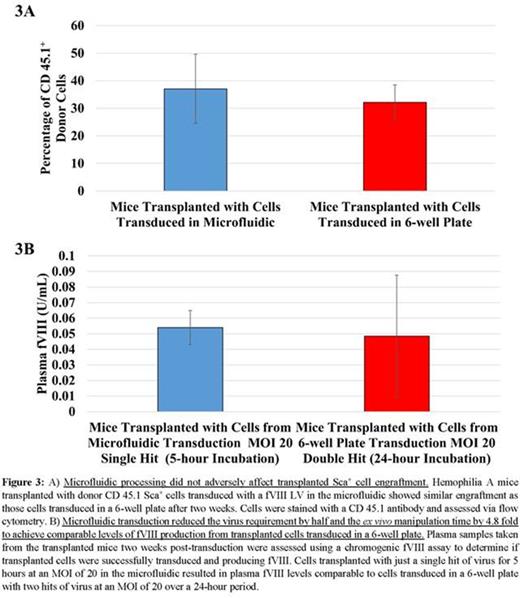
The high SA:volume ratio of the microfluidic enhances transduction by physically bringing cells and virus into closer proximity and enabling high concentrations of virus to be used without increasing the amount of virus set by the minimum volume requirements of LV transduction platforms (Fig. 1A). The polystyrene bottom of the microfluidic allows for Retronectin coating that immobilizes non-adherent cells on the bottom surface. LV can then be perfused at low concentrations to maintain a constant supply of fresh virus to the cells, increase convective mixing, and to minimize cell exposure to the toxic byproducts of LV production (Fig. 1B). These microfluidics have been scaled up to accommodate 106 cells, with potential to scale up to 107-108 cells (Fig. 1C). Cells transduced in the microfluidics showed 2-6 fold increases in GFP expression over well plates utilizing the same amount of cells, virus, and incubation times (Fig. 2A). The kinetics of LV transduction in the microfluidics also are faster, as seen by the steeper transduction curve. Five hours of incubation in the microfluidic yielded comparable transduction to 24 hours in the 6-well plate (Fig. 2B). Improvements in transduction also were observed by perfusing virus despite using lower virus concentrations (Fig. 2C). Finally, hemophilia A mice transplanted with donor CD45.1 Sca+ cells transduced in the microfluidic have engrafted (Fig. 3A) and produce fVIII (Fig. 3B) after two weeks with similar profiles to control cells transduced in a 6-well plate despite using half the amount of virus and shorter incubation times.
Conclusions and ongoing efforts: We describe a novel microfluidic that significantly reduces the amount of virus and ex vivo processing time required for therapeutic levels of transduction in clinical gene therapy. This device is versatile in its compatibility with current transduction strategies such as Retronectin and polybrene in addition to offering new approaches to boosting gene transfer efficiency. Furthermore, we have shown that the device has clinical potential by successfully scaling up cell numbers and transplanting mice with microfluidic transduced cells, of which there is an ongoing effort to monitor fVIII production and determine virus copy number. Future work will involve optimization with transduction-enhancing compounds, further scaling, and continued in vivo experiments.
Spencer:Expression Therapeutics: Equity Ownership. Doering:Bayer Healthcare: Consultancy, Honoraria, Research Funding; Expression Therapeutics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal