Abstract
Background: T-cell depleted haplo-HSCT is an established treatment for children with primary immune deficiencies (PID). However, children given this type of allograft are exposed to the risk of fatal events due to viral infections because of the prolonged impairment of adaptive immunity. We recently developed a novel method of selective T-cell depletion based on physical elimination of α/β T cells (ClinicalTrial.gov identifier: NCT01810120), which was shown to be safe and more effective than transplantation of positively-selected CD34+ cells for preventing life-threatening infections. However, we recorded some severe and even fatal viral infections, which prompted us to explore innovative approaches to accelerate the recovery of adaptive immunity. For this purpose, we designed an ongoing phase I/II trial aimed at testing the safety and the efficacy of post-transplant infusion of BPX-501 cells in children with malignant or non-malignant disorders (ClinicalTrials.gov identifier: NCT02065869). We report 3 cases of children with either severe combined immune deficiency (SCID) or Wiskott-Aldrich syndrome (WAS), who were enrolled in the dose escalation phase of the study and who cleared cytomegalovirus (CMV) or Adenovirus (AdV) infections likely due to the contribution of the BPX-501 cells.
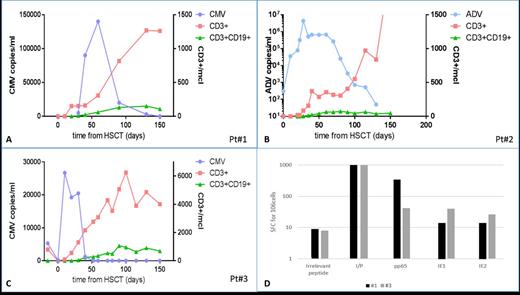
Patients and methods: Patient #1, affected by SCID, was transplanted from the HLA-haploidentical father. Before transplantation she had CMV-DNAemia which was treated with ganciclovir until donor stem cell infusion. She was given 2.5 x 105/kg BPX-501 cells on day 17 after transplantation. Patient #2, also affected by SCID, was transplanted from the HLA-haploidentical mother. Before transplantation she had AdV-DNAemia and high load of the virus in stools. She was given 5 x 105/kg BPX-501 cells on day 15 after transplantation. Patient #3 was affected by WAS and referred to the transplant unit; in the months preceding haplo-HSCT the child had developed CMV retinitis and hepatitis with high levels of CMV-DNAemia. This patient was transplanted from the father and received 1 x 106/kg BPX-501 cells on day 15 after haplo-HSCT. Basic phenotype of circulating lymphocytes was assessed by flow cytometry on fresh heparinized peripheral blood samples at 10, 20, 30, 60, 90, 120 and 150 days post haplo-HSCT, respectively. Since BPX-501 cells are CD3+/CD19+, it was easy to track the presence of these genetically modified cells. CMV specific reconstitution was also monitored through the INF gamma ELISPOT assay. In particular, peripheral blood mononuclear cells were stimulated for 16hrs in the presence of peptide libraries derived from pp65, IE1 and IE2 CMV-specific antigens.
Results: The increase in the number of both CD3+ T lymphocytes and BPX-501 cells over time after transplantation together with the modifications of DNAemia of both CMV and AdV in the 3 patients are reported in Panel A, B and C, respectively, of Figure 1. In all of these patients, the pre-existing viral infection was progressively cleared once the BPX-501 cells were infused. These cells expanded in vivo and are still persisting, contributing to the recovery of adoptive immunity. The median time to reach an absolute number of α/β CD3+ cells greater than 0.5x109/L was 90, 90 and 30 days, respectively. None of these patients experienced either acute or chronic Graft-versus-Host Disease (GvHD) and no organ inflammatory-related toxicity was recorded. All children are alive and disease free, without infections, at day +200, +180 and +160, respectively. The 2 patients with CMV infection showed a specific response for at least one CMV-derived antigen; indeed, one patient showed a prevalence in pp65 response, whereas in the second one, we observed a specific anti-CMV response against all three tested antigens (Figure 1 - Panel D).
Conclusions: Infusion of BPX-501 cells is able to accelerate the recovery of adaptive T-cell immunity in children with PID given haplo-HSCT after depletion of α/β T cells, thus rendering the procedure safer even in children with active infections at time of transplantation. These cells, once infused, expand in vivo and persist over time, contributing to the clearance of viral infections, without inducing GvHD.
Moseley:Bellicum Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal