Abstract
Background: The initial therapies for multiple myeloma have changed significantly in the recent years with routine incorporation of combinations containing IMiDs and proteasome inhibitors. However, patients with MM invariably relapse after their initial treatment and undergo successive therapies for continued control of their disease. The increasing access to new therapies for treatment of relapsed disease has led to improved survival of patients with MM. Many recent phase 3 trials have studied the impact of new agents on outcome of patients with MM who are relapsing after the initial lines of therapy for relapsed disease. However, the natural history of relapsed myeloma earlier in the course of relapsed disease has not been studied in detail.
Patients and methods: We designed a global, multicenter, retrospective study to examine the current treatment approaches for initial relapses in MM and patient outcomes following application of successive treatment regimens. Patients with multiple myeloma who experience a first relapse between January 1, 2007 and December 31, 2011 were identified at participating sites. Clinical and laboratory data pertaining to the time of diagnosis and from the time of individual relapses up to and including the third relapse will be obtained from the clinical records. The first date of treatment with an anti-myeloma regimen for first disease relapse was termed time zero (T0).
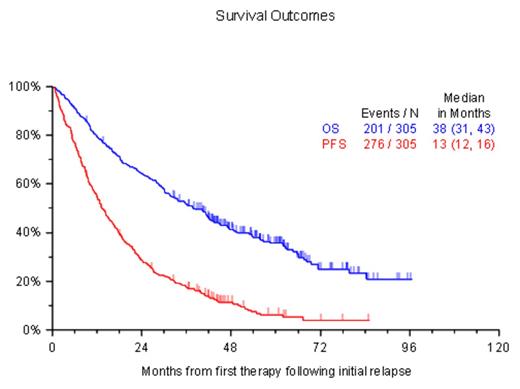
Results: Three hundred and fifteen patients were enrolled; median age at diagnosis was 59 years (range: 35 - 86), 59% were male. Patients were enrolled from North America (33%), Europe (35%) or Asia (32%). The patients were diagnosed between 1997 and 2011 and the median time to T0 from diagnosis was 23.9 months (range: 0.8 - 131.9 months). An IMiD (thalidomide or lenalidomide), bortezomib or alkylator (melphalan or cyclophosphamide) was part of the initial therapy in 63%, 36% and 51% of patients, respectively; 93 (33%) patients had stem cell transplantation as part of initial treatment. The median follow up from the study entry was 56 months; 117 were alive at the time of data extraction. A second line, third line and fourth line of treatment were recorded in 307, 163 and 84 patients respectively. An IMiD (thalidomide, lenalidomide, or pomalidomide), proteasome inhibitor (bortezomib or carfilzomib) or alkylator (melphalan or cyclophosphamide) was part of the therapy for first relapse in 50%, 59% and 33% of patients, respectively. The responses to the regimens after T0, the TTNT, PFS and OS to each line of therapy are provided in the Table.
Conclusion: The current study provides an assessment of the current treatment approaches used for initial treatment of MM and the salvage treatment options utilized. The study again demonstrates the progressively shorter duration of disease control with each successive treatment regimen, reflecting ongoing development of drug resistance. The overall survival of over 3 years from the time of therapy for first relapse highlights the impact of the newer therapies that have been introduced for this disease.
Treatment outcomes after initial relapse
| Endpoint . | 2nd line therapy N=307 . | 3rd line therapy N=163 . | 4th line therapy N=84 . |
|---|---|---|---|
| Partial response or better | |||
| Median in Months (95% Confidence Interval) | |||
| Overall Survival | 36.1 (30.2, 42.4) | 18.4 (14.5, 26.8) | 12.6 (10.4, 19.0) |
| Progression-Free Survival | 13.4 (11.8, 15.8) | 8.3 (6.5, 11.2) | 6.4 (5.0, 8.1) |
| Time to Next Treatment | 15 (12,17) | 9.8 (7.4, 12.0) | 6.6 (5.8, 8.7) |
| Endpoint . | 2nd line therapy N=307 . | 3rd line therapy N=163 . | 4th line therapy N=84 . |
|---|---|---|---|
| Partial response or better | |||
| Median in Months (95% Confidence Interval) | |||
| Overall Survival | 36.1 (30.2, 42.4) | 18.4 (14.5, 26.8) | 12.6 (10.4, 19.0) |
| Progression-Free Survival | 13.4 (11.8, 15.8) | 8.3 (6.5, 11.2) | 6.4 (5.0, 8.1) |
| Time to Next Treatment | 15 (12,17) | 9.8 (7.4, 12.0) | 6.6 (5.8, 8.7) |
Kumar:Onyx: Consultancy, Research Funding; Skyline: Consultancy, Honoraria; BMS: Consultancy; Sanofi: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Dimopoulos:Novartis: Honoraria; Amgen: Honoraria; Janssen-Cilag: Honoraria; Genesis: Honoraria; Janssen: Honoraria; Onyx: Honoraria; Celgene: Honoraria. Leleu:Janssen: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Amgen: Honoraria; TEVA: Honoraria; Novartis: Honoraria; BMS: Honoraria; Pierre Fabre: Honoraria; LeoPharma: Honoraria; Chugai: Honoraria. Moreau:Celgene, Janssen, Takeda, Novartis, Amgen: Membership on an entity's Board of Directors or advisory committees. Lahuerta:Janssen Cilag, Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vij:Takeda, Onyx: Research Funding; Celgene, Onyx, Takeda, Novartis, BMS, Sanofi, Janssen, Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal