Abstract
Background: Myelodisplastic syndrome (MDS) and acute myeloid leukemia (AML) are usually sporadic diseases. However, rare familial cases have helped to identify human disease genes and provided insight into hematopoiesis and leukemogenesis. Further, the ability to identify familial MDS/AML disease genes has improved due to advances in next generation sequencing technology. In addition to RUNX1, GATA2, CEBPA, germline mutations in both ETV6 and DDX41 were recently shown to predispose to myeloid hematologic malignancies.
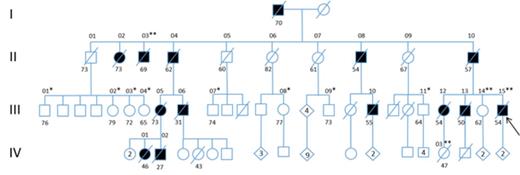
Methods: We investigated a large family with an inherited MDS/AML marked by erythroid hyperplasia in all affected individuals. A family pedigree was consistent with an autosomal dominant mode of inheritance with high penetrance. Whole exome sequencing was performed on germline DNA of the proband and multiple unaffected family members in order to identify the inherited genetic mutation predisposing to disease. Candidate single nucleotide variants (SNVs) were obtained after excluding all known SNVs with a variant allele frequency greater than 0.01, then prioritized and screened using DNA from a second affected individual.
Results: An Ala1337Thr missense variant in the ERBB3 gene, a member of the EGFR tyrosine kinase receptor family, was identified as the potential pathologic germline mutation in this family. Further sequencing identified the presence of this variant in two additional affected individuals and its absence in eight unaffected family members, indicating that it co-segregates with disease. Functional validation of this ERBB3 variant was performed using an erythroleukemia cell line. Overexpression of the ERBB3 A1337T mutant in TF1 cells conveyed a block in erythroid differentiation compared to cells expressing unmutated ERBB3, measured by the lack of upregulation of Glycophorin A expression in the presence of erythropoietin. This activity was dependent upon the presence of Neuregulin-1β, the ERBB3 receptor ligand, and expression of the co-receptor ERBB2. In addition, ERBB3 mutated cells displayed a 1.3-fold growth advantage over unmutated ERBB3 in the presence of the Neuregulin-1β.
Conclusions: This work has identified a novel mutation that predisposes to MDS/AML and adds ERBB3 to the growing list of disease predisposition genes in hematologic malignancies. It will allow for the early diagnosis of individuals with inherited erythroid MDS/AML prior to the onset of disease. In addition, it allows for the characterization of a gene with a likely crucial role in erythropoiesis as well as hematologic disease.
Brodsky:Alexion Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal