Abstract
Acute myeloid leukemia (AML) is one of the most common and fatal forms of hematopoietic malignancies. With standard chemotherapies, only 30-50% of younger (aged <60) and 5-10% of older patients with AML survive longer than 5 years. Aberrancy of FMS-like tyrosine kinase 3 (FLT3) occurs in the majority cases of AML. Two major classes of constitutively activating mutations of FLT3, i.e. internal-tandem duplications (ITDs) and tyrosine kinase domain (TKD) point mutations are found in more than 30% of AML cases and usually predict poor prognosis. Overexpression of FLT3 has also been reported in more than 70% of AML cases with a variety of AML subtypes, e.g. MLL (Mixed Lineage Leukemia)-rearranged or FLT3 -ITD AML, and may be associated with poor survival in AML patients. Given the disappointing results with FLT3 tyrosine kinase inhibitors (TKIs) in clinical trials in the past decade, decreasing the overall abundance of FLT3 at the RNA and protein levels would be an alternative strategy to treat AMLs with FLT3 overexpression and/or FLT3 -ITD/TKD mutations.
MicroRNAs (miRNA) are a class of small, non-coding RNAs that play important roles in post-transcriptional gene regulation. We recently reported that miR-150 functions as a pivotal tumor-suppressor gatekeeper in MLL-rearranged and other subtypes of AML, through targeting FLT3 and MYB directly, and the MYC/LIN28/HOXA9/MEIS1 pathway indirectly. Our data showed that MLL-fusion proteins up-regulate FLT3 level through inhibiting the maturation of miR-150. Therefore, our findings strongly suggest a significant clinical potential of restoration of miR-150 expression/function in treating FLT3 -overexpressing AML.
In the present study, we first analyzed FLT3 expression patterns and prognostic impact in a large cohort of AML patients (n=562). We found that FLT3 is aberrantly highly expressed in FAB M1/M2/M5 AML or AML with t(11q23)/MLL -rearrangements, FLT3 -ITD or NPM1 mutations, and that increased expression of FLT3 is an independent predictor of poor prognosis in patients with FLT3 -overexpressing AML.
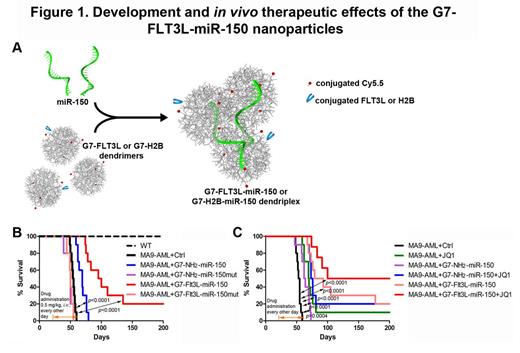
To treat FLT3 -overexpressing AML, we developed a novel targeted nanoparticle system consisting of FLT3 ligand (FLT3L)-conjugated G7 poly(amidoamine) (PAMAM) dendriplexes encapsulating miR-150 oligos (see Figure 1A). In FLT3 -overexpressing cell lines, the uptake ratios of the G7-FLT3L dendrimers were much higher (50.3~97.1%) than the G7-histone 2B (H2B) control nanoparticles (4.3~33.2%). And the uptake only took minutes. By integrating the miR-150 oligo with G7-FLT3L dendrimers, we constructed the G7-FLT3L-miR-150 dendriplexes, which significantly reduced the viability and increased the apoptosis of MONOMAC-6 cells carrying t(9;11) in a dose-dependent manner. To increase the stability of miR-150 oligos, we incorporated a 2'-o -methyl (2'-O Me) modification into the miRNA oligos. Indeed, the G7-FLT3L nanoparticles carrying 2'-O Me modified miR-150 exhibited a more sustained inhibition on cell growth.
In order to further investigate the in vivo therapeutic effects of the miR-150 nanoparticles, we used a MLL -rearranged leukemia model. We transplanted wild-type recipient mice with primary mouse leukemic cells bearing the MLL-AF9 fusion. After the onset of leukemia, the mice were treated with G7-Flt3L or G7-NH2 control nanoparticles complexed with 2'-O Me-modified miR-150 oligos. In these treated animals, G7-Flt3L-miR-150 nanoparticles tended to be enriched in the bone marrow. The G7-Flt3L-miR-150 nanoparticles showed the best therapeutic effect (with median survival of 86 days), as compared with the control group (Ctrl; PBS treated; with median survival of 54 days) or the G7-NH2-miR-150 treated group (with median survival of 63 days). Nanoparticles carrying miR-150 mutant oligos showed no anti-leukemia effect at all. Notably, the G7-Flt3L-miR-150 treatment almost completely blocked MLL-AF9 -induced leukemia in 20% of the mice (Fig. 1B). Furthermore, the G7-Flt3L-miR-150 nanoparticles showed a synergistic effect with JQ1, a small-molecule inhibitor of the MYC pathway, in treating AML in vivo (Fig. 1C).
Collectively, we have developed a novel targeted therapeutic strategy to treat FLT3-overexpressing AML, such as MLL-rearranged leukemias, which are resistant to currently available therapies, with both high specificity and efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal