Abstract
Introduction: ASP2215 is a highly selective inhibitor of AXL and FMS-like tyrosine kinase-3 (FLT3) receptors. ASP2215 is active against both FLT3 internal tandem duplication [ITD] and D835 mutations. Prior analyses of an open-label, dose-escalation/dose-expansion study in subjects with relapsed or refractory acute myeloid leukemia (R/R AML) show ASP2215 was well tolerated from 20-300 mg and associated with antileukemic activity in FLT3 mutation-positive (FLT3+) patients at ≥80 mg with minimal activity observed in wild-type FLT3 subjects. Here we describe the tolerability and potent activity of ASP2215 in a large cohort of FLT3+ patients.
Methods: Patients (≥18 years) with R/R AML were assigned to treatment in dose-escalation cohorts or were randomized to an open dose level in the dose-expansion cohorts. Although FLT3 mutation was not an inclusion criterion, each expanded dose level enrolled ≥10 FLT3+ subjects; 120 mg and 200 mg dose levels were further expanded with ≥40 FLT3+ subjects. Tolerability was assessed by adverse event (AE) monitoring. Response assessment was based on modified Cheson criteria and duration of response and overall survival were calculated using Kaplan-Meier estimates.
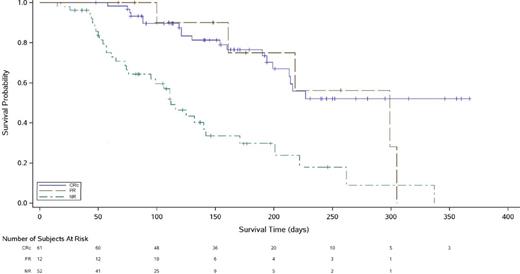
Results: As of June 19, 2015,215 patients with a median age of 61 yr (range: 21-90) had received ≥1 dose of ASP2215 (safety population). Across the safety population, 65% of subjects received ≥2 prior lines of AML therapy, 29% had a hematopoietic stem cell transplant prior to ASP2215 treatment, and 23% had prior tyrosine kinase inhibitor (TKI) treatment. Approximately 73% of patients were FLT3+, of which 137 had FLT3-ITD mutation, 7 were FLT3-D835+, and 9 had both FLT3-ITD and D835. Treatment-related AEs of all Grades, reported in ≥10% of the safety population were diarrhea (16%), fatigue (13%), and increased AST (11%); <2% of subjects reported a Grade ≥3 QTc prolongation. Activity was assessed in the 133 FLT3+ patients treated with ASP2215 ≥80 mg. Overall response rate (ORR; composite complete [CRc] plus partial remission [PR]) for all FLT3+ subjects was 55% (Table). Median overall survival for FLT3+ patients receiving ASP2215 ≥80 mg was ~29 weeks (95% CI: 22-32) and was similar for patients who achieved CRc or PR (Figure). Treatment with ≥80 mg ASP2215 was associated with an ORR of 60% in FLT3-ITD subjects; ORR for the other FLT3 populations was 29% (Table). No difference was observed in median ORR of ASP2215 (≥80 mg) in TKI-naïve patients (55%) and patients with prior TKI treatment (55%).
Conclusions: ASP2215, a novel AXL/FLT3 TKI, was well tolerated in subjects with R/R AML and demonstrated a strong antileukemic activity in FLT3+ subjects. Importantly, the ASP2215 response rate in these FLT3+ patients was independent of prior TKI treatment. Even in this heavily pretreated population, the survival of R/R FLT3+ AML patients who received ≥80 mg ASP2215 was longer than prior reports of cytotoxic chemotherapy or other FLT3 inhibitors.
ASP2215 Response Assessment
| . | 80 mg . | 120 mg . | 200 mg . | 300 mg . | 450 mg . | Total . |
|---|---|---|---|---|---|---|
| All FLT3+ Subjects | ||||||
| Population, n | 12 | 52 | 57 | 10 | 2 | 133 |
| CRc, n (%) | 5 (42) | 25 (48) | 28 (49) | 3 (30) | 0 | 61 (46) |
| PR, n (%) | 3 (25) | 3 (6) | 3 (5) | 3 (30) | 0 | 12 (9) |
| ORR, n (%) | 8 (67) | 28 (54) | 31 (54) | 6 (60) | 0 | 73 (55) |
| Subjects with FLT3-ITD Only | ||||||
| Population, n | 10 | 46 | 50 | 8 | 0 | 114 |
| CRc, n (%) | 4 (40) | 23 (50) | 26 (52) | 3 (38) | 0 | 56 (49) |
| PR, n (%) | 3 (30) | 3 (7) | 3 (6) | 3 (38) | 0 | 12 (11) |
| ORR, n (%) | 7 (70) | 26 (57) | 29 (58) | 6 (75) | 0 | 68 (60) |
| Subjects with FLT3-D835 and Subjects with FLT3-ITD and FLT3-D835 | ||||||
| Population, n | 2 | 5 | 5 | 1 | 1 | 14 |
| CRc, n (%) | 1 (50) | 1 (20) | 2 (40) | 0 | 0 | 4 (29) |
| PR, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| ORR, n (%) | 1 (50) | 1 (20) | 2 (40) | 0 | 0 | 4 (29) |
| . | 80 mg . | 120 mg . | 200 mg . | 300 mg . | 450 mg . | Total . |
|---|---|---|---|---|---|---|
| All FLT3+ Subjects | ||||||
| Population, n | 12 | 52 | 57 | 10 | 2 | 133 |
| CRc, n (%) | 5 (42) | 25 (48) | 28 (49) | 3 (30) | 0 | 61 (46) |
| PR, n (%) | 3 (25) | 3 (6) | 3 (5) | 3 (30) | 0 | 12 (9) |
| ORR, n (%) | 8 (67) | 28 (54) | 31 (54) | 6 (60) | 0 | 73 (55) |
| Subjects with FLT3-ITD Only | ||||||
| Population, n | 10 | 46 | 50 | 8 | 0 | 114 |
| CRc, n (%) | 4 (40) | 23 (50) | 26 (52) | 3 (38) | 0 | 56 (49) |
| PR, n (%) | 3 (30) | 3 (7) | 3 (6) | 3 (38) | 0 | 12 (11) |
| ORR, n (%) | 7 (70) | 26 (57) | 29 (58) | 6 (75) | 0 | 68 (60) |
| Subjects with FLT3-D835 and Subjects with FLT3-ITD and FLT3-D835 | ||||||
| Population, n | 2 | 5 | 5 | 1 | 1 | 14 |
| CRc, n (%) | 1 (50) | 1 (20) | 2 (40) | 0 | 0 | 4 (29) |
| PR, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| ORR, n (%) | 1 (50) | 1 (20) | 2 (40) | 0 | 0 | 4 (29) |
CRc, composite complete remission (complete remission + complete remission with incomplete platelet recovery + complete remission with incomplete hematologic recovery); ORR, overall response rate; PR, partial response.
NR, no response. Subjects with non-evaluable data (N=8) were not included in this curve.
Overall Survival by Best Overall Response Achieved with ASP2215 ≥80 mg Across All FLT3+ Subjects
Overall Survival by Best Overall Response Achieved with ASP2215 ≥80 mg Across All FLT3+ Subjects
Altman:BMS: Other: Advisory board; Novartis: Other: Advisory board; Spectrum: Other: Advisory board; Ariad: Other: Advisory board; Seattle Genetics: Other: Advisory board; Astellas: Other: Participation in an advisory board December 2013. Off Label Use: ASP2215 is currently under investigation for the treatment of AML and is not yet approved.. Perl:Arog Pharmaceuticals: Consultancy; Asana Biosciences: Consultancy; Actinium Pharmaceuticals: Consultancy; Ambit/Daichi Sankyo: Consultancy; Astellas US Pharma Inc.: Consultancy. Cortes:Pfizer: Consultancy, Research Funding; BerGenBio AS: Research Funding; Novartis: Consultancy, Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy. Levis:Arog: Research Funding; Ambit: Research Funding; Takeda: Research Funding; Astellas: Consultancy. Smith:Plexxikon: Research Funding; Astellas: Research Funding. Claxton:NCI: Research Funding; Medimmune, Inc: Research Funding; Ambit Biosciences Corp: Research Funding; Incyte Corporation: Research Funding; Merck Sharp & Dohme Corp: Research Funding; Astellas Pharma: Research Funding. Erba:Seattle Genetics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Ariad: Consultancy; Celgene: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; GlycoMimetics: Other: Data Safety and Monitoring Committees ; Jannsen (J&J): Other: Data Safety and Monitoring Committees ; Seattle Genetics: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Amgen: Consultancy, Research Funding; Celator: Research Funding; Millennium/Takeda: Research Funding; Astellas: Research Funding; Sunesis: Consultancy; Celator: Research Funding; Pfizer: Consultancy; Astellas: Research Funding; Daiichi Sankyo: Consultancy; Sunesis: Consultancy; Ariad: Consultancy; Pfizer: Consultancy; GlycoMimetics: Other: Data Safety and Monitoring Committees ; Jannsen (J&J): Other: Data Safety and Monitoring Committees ; Daiichi Sankyo: Consultancy. Gill:Astellas Pharma US, Inc: Employment. Goldberg:Cyclacel: Research Funding; Celetor: Research Funding; Pfizer: Research Funding; Ambit: Research Funding; Astellas: Research Funding. Jurcic:Astellas Pharma: Research Funding. Larson:Astellas: Consultancy, Research Funding. Lui:Astellas Pharma US, Inc: Employment. Ritchie:Incyte: Speakers Bureau; Novartis: Speakers Bureau; Ariad: Other: Advisory Board; Celgene: Speakers Bureau; Onyx: Speakers Bureau. Sargent:Astellas Pharma US, Inc: Employment. Schiller:Sunesis: Honoraria, Research Funding. Strickland:Sunesis Pharmaceuticals: Other: Steering Committee and Advisory Board Participation; Alexion Pharmaceuticals: Other: Advisory Board Particpation; Amgen: Other: Advisory Board Particpation; Daiichi-Sankyo: Other: Advisory Board Particpation; Boehringer-Ingelheim: Other: Advisory Board Particpation. Wang:Immunogen: Research Funding. Stuart:Sunesis: Honoraria, Other: Advisory Board, Research Funding; Astellas Pharma, Inc: Research Funding. Baldus:Novartis: Research Funding. Martinelli:MSD: Consultancy; ARIAD: Consultancy; BMS: Speakers Bureau; Pfizer: Consultancy; Novartis: Speakers Bureau; Roche: Consultancy. Bahceci:Astellas Pharma Global Development: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal