Abstract

INTRODUCTION: T-prolymphocytic leukemia (T-PLL) is a rare T cell malignancy with an aggressive clinical course. Preliminary clinical data suggest that allogeneic stem cell transplantation (alloSCT) may provide long-term disease control in a proportion of patients. However, direct evidence that graft-versus-leukemia (GVL) activity is indeed effective in T-PLL is lacking. We sought to investigate GVL in T-PLL by correlating minimal residual disease (MRD) kinetics with immune modulatory interventions (immunosuppression tapering, donor lymphocyte infusions (DLI), chronic graft-versus-host disease (cGvHD)), and T cell receptor (TCR) repertoire diversity alterations after alloSCT.
METHODS: The study sample consisted of 10 consecutive patients who received alloSCT for T-PLL at the University of Heidelberg between 2007 and 2015. Quantitative MRD monitoring was performed using clone-specific real-time quantitative PCR (RQ-PCR) of clonal TCR beta (TRB) and/or gamma (TRG) gene rearrangements. Data interpretation followed EuroMRD guidelines. In selected patients, TCR repertoire diversity was analyzed longitudinally by next-generation sequencing (NGS). TRBV-TRBD-TRBJ gene rearrangements were amplified according to BIOMED2 protocol on genomic DNA. PCR products were sequenced on Illumina's MiSeq platform. NGS data were analyzed through a purpose-built bioinformatics immunoprofiler (EuroClonality-NGS consortium). Rearrangements with very similar junctional amino acid sequences and identical TRBV and TRBJ gene usage were defined as clonotypes.
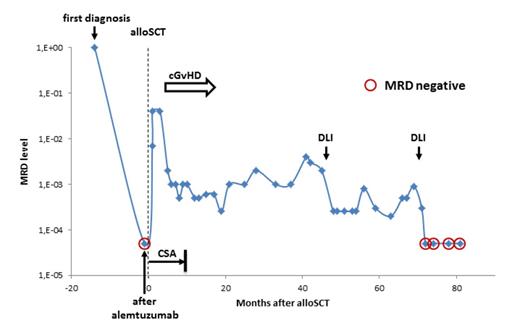
RESULTS: Patients underwent alloSCT in remission after first-line (8) or salvage (2) alemtuzumab-based therapy. 5 patients were allografted with an unrelated donor, 4 with a related donor, and one received haploidentical alloSCT. Conditioning was fludarabine with cyclophosphamide and/or total body irradiation-based. All patients had a cytological complete response (CR) after alloSCT. 2 patients died early because of acute GvHD, and one had no MRD marker, leaving 7 patients for MRD monitoring. Of these, 3 were MRD- at alloSCT, whereas 5 patients remained or became MRD+ early after alloSCT. In all of these 5 patients, immunosuppression tapering (3) or DLI (2) resulted in significant reduction of MRD levels (range 1-3 log) and was accompanied by cGvHD in 3 patients. However, durable MRD- was obtained in only 2 patients (alive 81+ and 12+ months post transplant), whilst MRD re-increased in 3 patients after 5-28 months despite ongoing cGvHD in one of them. NGS of the TCR repertoire was performed longitudinally in 3 patients with the longest follow-up. A total of 104 samples (blood (n=91) and BM (n=10) plus 3 donor blood samples) were sequenced. The sample at diagnosis showed one or two major clonotypes in all 3 sequenced T-PLLs, predominantly reflecting a mono- or biallelic leukemic TRB gene rearrangement. Kinetics of this leukemic clonotype followed kinetics of RQ-PCR MRD measurement, demonstrating that NGS can be used to quantify MRD in T cell lymphoma. Immediately after transplantation, the TRB repertoire was heavily skewed in all 3 patients, but recovered over time. Also in all 3 patients, MRD responses were reproducibly associated with a shift from a clonal, T-PLL-driven profile to a polyclonal signature. This corresponded to the donor clonotype repertoire and disappeared with increasing MRD levels. In each of the samples after alloSCT, several expanded non-leukemic clonotypes were observed at a maximum frequency from 3% to 54% of total TRB sequences. However, during MRD response, novel dominant clonotypes that could explain a clonal GVL effect did not emerge. Figure 1 shows an example of a patient repeatedly showing MRD response to immune interventions. Currently, 5 patients are alive and in cytological CR (4-81 months after alloSCT), translating into a median relapse-free survival of 40 months.
CONCLUSIONS: The MRD responses to immune interventions observed here provide the first direct evidence for the efficacy of GVL in T-PLL. However, the GVL effect in T-PLL appears to be often only limited or transient. It also does not seem to be caused by the emergence of novel dominant T cell clones but is rather relying on a poly-/oligoclonal T cell response.
Example for MRD kinetics in relation to immune interventions.
Example for MRD kinetics in relation to immune interventions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal