Abstract
Introduction: Proof of principle that adoptively transferred NK cells can mediate regression of hematological malignancies has recently been established in the clinic. Despite recent advances in the field, the overall efficacy of NK-cell based immunotherapy remains limited. Directing cellular migration to tumor-bearing tissues could be used as a method to improve the efficacy of NK cell-based immunotherapy. As most hematological malignancies arise from bone marrow (BM) compartments, we investigated the potential of genetic modification of NK cells to express high levels of the BM homing chemokine receptor CXCR4 to improve NK cell migration to BM compartments in vivo.
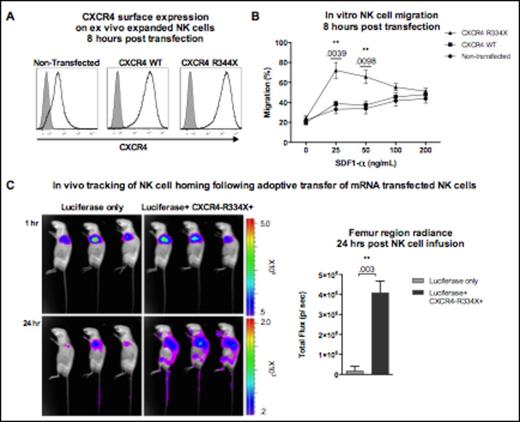
Methods: Human NK cells were expanded ex vivo in G-rex flasks for 14 days using irradiated EBV-LCL feeder cells and IL-2 containing media. Electroporation (EP) of NK cells with mRNA coding for the wild-type CXCR4 receptor (WT CXCR4) and the gain-of-function mutation CXCR4 receptor (CXCR4-R334X) was performed using the MaxCyte GT instrument. Cell viability and receptor expression was assessed by flow cytometry using a BD LSR II Fortessa. In vitro transwell migration assays towards the CXCR4 ligand SDF-1α were performed in serum-free media over 2 hours at +37¡C. Pretreatment of NK cells with 100 uM of plerixafor for 30 min at +4¡C prior to migration assays was used for CXCR4 blockade experiments. In vivo homing studies were performed with bioluminescence tracking of luciferase-transfected NK cells in NSG mice. Animals were imaged using an IVIS Bioluminescence imager 1 and 24 hours after adoptive NK cell transfer.
Results: EP of ex vivo expanded NK cells with either WT CXCR4 or CXCR4-R334X mRNA both resulted in a substantial increase in CXCR4 surface expression for up to 36 hours compared to non-EP NK cell controls. In vitro assays showed CXCR4-R334X transfected NK cells had superior migration to SDF-1α compared to both WT CXCR4 transfected and control NK cells, with an average 40% increase in their migration capacity towards SDF-1α compared to non-transfected NK cells (n=10 donors). This augmented migration capacity was abrogated when the CXCR4 receptor was selectively blocked with plerixafor. To confirm that CXCR4-R334X modified NK cells had improved BM homing capacity in vivo, we compared the distribution of these cells using bioluminescent imaging (BLI) after transfection with luciferase mRNA and intravenous injection into NSG mice (n=3). Twenty-four hours after adoptive transfer, CXCR4-R334X mRNA EP NK cells had improved homing to BM compartments such as the vertebrae, sternum ribs, and femurs compared to their unmodified NK cells counterparts (figure).
Conclusions: The data demonstrate that genetic modification of NK cells with CXCR4-R334X mRNA can be utilized to efficiently direct their homing of infused NK cells to BM compartments in vivo. We hypothesize that CXCR4-modified NK cells can be utilized to improve the efficacy of adoptive NK cell immunotherapy for patients with BM-residing malignancies such as leukemia and multiple myeloma.
Emily R. Levy is a predoctoral candidate in the Molecular Medicine program of Institute for Biomedical Sciences at the George Washington University. This work is from a dissertation to be presented to the above program in partial fulfillment of the requirements for the Ph.D. degree.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal