Abstract
Introduction:
Umbilical cord blood (UCB) serves as a suitable donor source in hematopoietic cell transplantation. However, the initial time to engraftment in UCB transplantation (UCBT) is delayed because of its insufficient graft cell numbers, which often limits its use in UCBT. According to the report by Japanese UCB bank, the majority of UCB units remain unused clinically because of their low graft cell doses. Therefore, we sought to develop a new strategy to improve outcomes of UCBT by using multiple (more than 3) units to augment graft cell doses. We reported at the ASH meeting 2014 that an early hematopoietic recovery in lethally irradiated mice was observed by combining hematopoietic stem/progenitor cells (HSC/HSPCs) derived from multiple allogeneic mouse strains. Herein to establish a clinically relevant procedure, we provide a proof of benefit of this approach using human UCB cells in xenotransplantation models.
Methods:
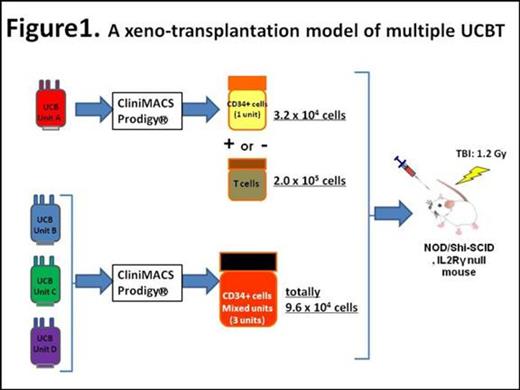
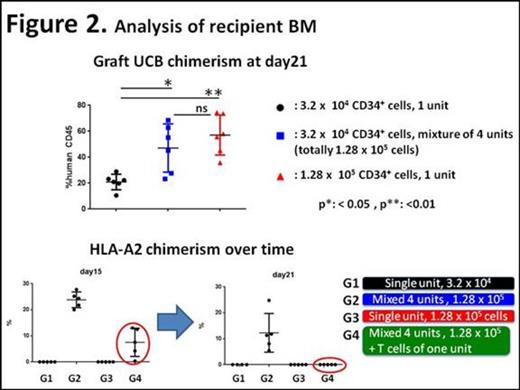
We initially subjected a single UCB (HLA-A2 negative) to CliniMACS Prodigy®(Miltenyi Biotec, Bergisch Gladbach, Germany) to acquire mononuclear cells (MNCs). Obtained MNCs were further processed for isolation of either CD34+ cells or CD3+ T cells using microbeads techniques (Figure 1, Unit A). We next combined 3 frozen UCB units (Units B-D, all HLA-A2 positive) into one bag after thawing and then applied them to CliniMACS Prodigy®. Mixed CD34+ cells were purified from obtained MNCs. These test CD34+ cells were transplanted into sublethally-irradiated immunodeficient mice. Mice in the first group received single unit CD34+ cells alone (Unit A, G1 in Figure 2, 3.2 x 104 cells). The additive effect on human cell engraftment was tested in mixed CD34+ cells by transplanting them (Units B-D, G2, 9.6 x 104 cells) together with the single unit CD34+ cells (Unit A, 3.2 x 104 cells). Another cohort of mice (G3) received Unit A CD34+ cells at a dose 4 times more than that in G1 (1.28 x 105 cells). We also tested whether the inclusion of CD3+ T cells (2 x 105 cells) derived from one UCB (Unit A) favored formation of single donor chimerism. Detailed donor cell chimerism was determined in bone marrow over time by flow cytometry analysis, using HLA subtypes as indicators of donor origins.
Results:
As formerly reported, in a multiple-donor transplantation model using mouse bone marrow cells, combined multiple units of allogeneic HSC/HSPCs were shown to protect lethally-irradiated recipient mice, and to accelerate the recovery of granulocytes, hemoglobin and platelets when infused additionally with a single graft that was composed of whole bone marrow cells. Single donor chimerism was achieved long-term in these mice. Experiments using sorted population of a graft demonstrated that an appropriate dose of T cells should be contained within the primary unit to achieve single donor chimerism through graft versus graft reactions. We then tested whether our strategy was applicable to human UCB cells in xeno-transplantation models.
At the first attempt, it was extremely difficult to extract viable CD34+ cells manually from frozen UCB units. To overcome this problem, we employed CliniMACS Prodigy®. Irrespective of HLA type disparities, we eventually succeeded in extracting viable MNCs from multiple UCB units at one time by using CliniMACS Prodigy®. Purified cells were obtained from them using microbeads techniques, with %CD34 and %CD3 of the final test cells being more than 98 % and 96 %, respectively. Up to 9 UCB units were applicable to CliniMACS Prodigy® together at once.
As shown in Figure 2, combined multiple UCB units exhibited robust hematopoietic reconstitution. The human cell chimerism in G2 (mixed units) was as high as that observed in G3 (single unit with the same dose). As evidenced by sequential chimerism analysis, inclusion of T cells in one graft achieved single donor chimerism following transient mixed donor chimerism.
Conclusions:
Our study uncovered that combined multiple UCB units of allogeneic HSC/HSPCs were capable of accelerating hematopoietic reconstitution when manipulated appropriately and added to an otherwise "insufficient dose" setting of transplantation both for mice and human cases. Development of a new strategy of multiple UCB transplantation will open a door for wider application of UCBT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal