Abstract
Prenatal transplantation capitalizes on the unique fetal environment, allowing for life-long engraftment of allogeneic stem cells without the need for harsh conditioning regimens. A prerequisite for stable engraftment of allogeneic cells likely requires the negative selection of donor-specific host effector T cells (Teff) and the support of donor-specific regulatory T cells (Tregs). However, little is known about the interplay between these cell types during development. The purpose of this study was to characterize the dynamic relationship between donor-specific Teff and Tregs as they emerge during development.
Prenatal allogeneic chimeras were established by in utero transplantation of E14 fetal liver light density cells into age-matched allogeneic fetal recipients (Balb/c to B6 or B6 to Balb/c). In this model, immature T cells from B6 mice expressing TCRv-beta-5, 11, and 12 are negatively selected by mtv-8 superantigen complexed with I-E class MHC II on Balb/c cells. As alpha/beta TCR rearrangement does not occur until E16, this established transplantation model allows for alloantigen to be present from the earliest stages of thymic selection. Kinetic analysis of donor-specific T cell populations was performed in peripheral blood paired with in depth analysis in thymus and spleen in control and chimeric mice.
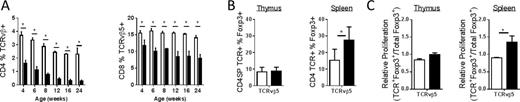
Negative selection of donor-specific Teff cells occurs at an unexpectedly slow pace in Balb/c to B6 prenatal chimeras. Donor-specific CD4 and CD8 Teff are significantly decreased in frequency at 4 weeks of age but do not reach maximal deletion until 12 weeks of age (TCRv-beta-5 data shown in Figure A). Further analysis demonstrated that this slow elimination of donor-specific Teff was paired with an early increase in the frequency of donor-specific Tregs at 4 weeks of age (TCRv-beta-5 data shown in Figure B.) This increase in donor-specific Tregs likely occurred as a result of peripheral expansion as there was no change in the frequency of donor-specific Tregs in the thymus (Figure B) and no change in the frequency of these cells that expressed the markers of thymically derived natural Tregs neuropilin-1 or helios (data not shown). In agreement with this hypothesis, donor-specific splenic Tregs incorporated BrdU at a higher rate than other Tregs in young mice indicating a potential expansion of donor-specific Tregs in the periphery (TCRv-beta-5 data shown in Figure C.)
Collectively, these data demonstrate that prenatal transplantation is characterized by: 1) a surprisingly slow reduction of donor-specific Teff subsets; 2) an early expansion of donor-specific Tregs. Further studies will explore the role of donor-specific Tregs in controlling early immunity to prenatally encountered antigens.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal