Abstract
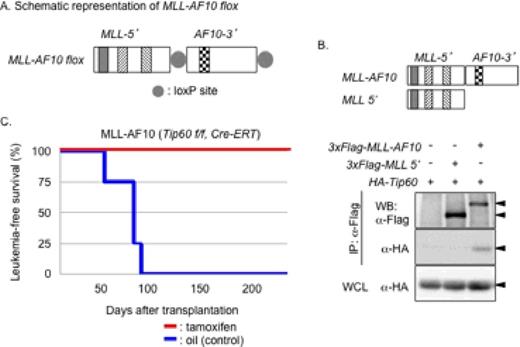
Chromosome translocation involving the mixed lineage leukemia (MLL) gene which generates an in-frame fusion gene of the MLL 5′-region and partner genes, is a common rearrangement in acute myeloid and lymphoid leukemia that is associated with poor prognosis. Knock-in and retroviral transduction studies show that MLL-fusion results in constitutive activation of the transcription of target genes such as Hoxa9 and Meis1 during the development of leukemia. Recent studies show that several transcription regulators, such as Dot1L, Cbx8, PAF1, and AEP/EAP complexes, are required for the leukemogenic activity of MLL-fusion; however, the underlying mechanisms remain elusive. To clarify the mechanism of epigenetic regulation by MLL-fusions, we established a novel leukemia model by generating a conditional MLL-AF10 fusion gene, MLL-AF10 flox, in which the 3′-AF10 region is deleted by 4-OHT-activated Cre-ERT recombinase, resulting in inactivation of MLL-AF10 flox (Figure 1A). Mouse hematopoietic stem/progenitor cells (c-kit+) were immortalized by retroviral transduction of MLL-AF10 flox and cultured in vitro or transplanted into irradiated recipient mice to induce AML in vivo. Treatment of MLL-AF10 flox cells with 4-OHT in vitro to inactivate MLL-AF10 flox downregulated Hoxa9 expression and markedly decreased colony-forming ability. In addition, the inactivation of MLL-AF10 flox rapidly decreased the acetylation level of the histone H2A variant H2A.Z on the Hoxa9 locus. These results suggest that MLL-AF10, possibly together with a histone acetyltransferase (HAT), regulates the acetylation of H2A.Z on the Hoxa9 locus. To identify the HAT responsible for H2A.Z acetylation induced by MLL-AF10, protein complexes associated with H2A.Z-containing nucleosomes were purified, resulting in the identification of Tip60, a MYST-type HAT in a complex with H2A.Z. MLL-AF10 physically interacted with Tip60 via the AF10 C-terminal portion of MLL-AF10 (Figure 1B). ChIP analysis showed that MLL-AF10 and Tip60 co-localize on the Hoxa9 locus in MLL-AF10-transformed cells (MLL-AF10 cells). Furthermore, conditional deletion of Tip60 in MLL-AF10 (Tip60 Flox/Flox, Cre-ERT2) cells dramatically downregulated Hoxa9 expression and resulted in the accumulation of unacetylated H2A.Z on the Hoxa9 locus. Consistent with these data, in vitro acetylation analysis showed that Tip60 directly acetylates H2A.Z. To assess the role of Tip60 in leukemia development in vivo, MLL-AF10 (Tip60 Flox/Flox, Cre-ERT2) leukemia cells were injected into recipient mice. Animals receiving intraperitoneal injection of tamoxifen to delete Tip60 failed to develop MLL-AF10 leukemia (Figure 1C). These data indicate that Tip60 is required for the development of MLL-AF10 leukemia and suggest that MLL-AF10 recruits Tip60 to acetylate H2A.Z on the Hoxa9 locus. The effect of H2A.Z acetylation on Hoxa9 expression was examined by purifying nucleosomes containing acetylation-deficient 3KR H2A.Z (which mimics unacetylated H2A.Z), in which lysines 4, 7, and 11 were substituted by arginine. 3KR H2A.Z preferentially formed nucleosomes with histone H3 trimethylation at lysine 27, which is catalyzed by polycomb repressive complex 2 (PRC2). This finding suggests that nucleosomes including unacetylated H2A.Z are the preferential targets of PRC2. Loss of Tip60 in MLL-AF10 cells resulted in decreased levels of acetylated H2A.Z on the Hoxa9 locus and the recruitment of Ezh2 (a catalytic subunit of PRC2) and increased histone H3 K27 trimethylation. Taken together, these data indicate that Tip60 is a critical factor in the development of MLL-AF10 leukemia. MLL-AF10 may maintain an active chromatin state on its target genesby recruiting Tip60, which acetylates H2A.Z to prevent PRC2 recruitment and gene silencing. On the other hand, unacetylated H2A.Z may be a signal for PRC2 recruitment, which would be induced as a result of Tip60 loss or inactivation of MLL-AF10.
Kitabayashi:Daiichi Sankyo Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal