Abstract
Introduction: Patients with chronic lymphocytic leukemia (CLL) may have reduced health-related quality of life (HRQL) such as severe fatigue during the course of their disease and its treatment. While current treatments aim to increase progression-free survival, it is also important to maintain good HRQL for as long as possible.
Methods: Ibrutinib, a first-in-class, once-daily, oral, covalent inhibitor of Bruton's tyrosine kinase, is approved in patients with previously treated CLL and in patients with CLL with del17p, and has recently been shown to reduce the risk of disease progression or death by 80% when added to standard chemoimmunotherapy with BR. In this randomized, double-blind, placebo-controlled, phase 3 study, where patients with active CLL following ≥ 1 prior therapy were randomized 1:1 to receive BR (≤ 6 cycles) with either ibrutinib (420 mg daily) (n = 289) or placebo (n = 289), HRQL and fatigue data were examined. Measures included the FACIT-Fatigue, EORTC QLQ-C30-CLL16, and EQ-5D-5L, which capture general physical and emotional impacts of disease and treatments as well as CLL-specific symptoms. Because examination of mean values may obscure meaningful changes in outcomes, fatigue scores were also examined by baseline quartile with respect to clinical, sociodemographic, and HRQL factors as well as for change over time. Individual CLL symptom items were also examined. The association between changes in hemoglobin levels and fatigue was also examined.
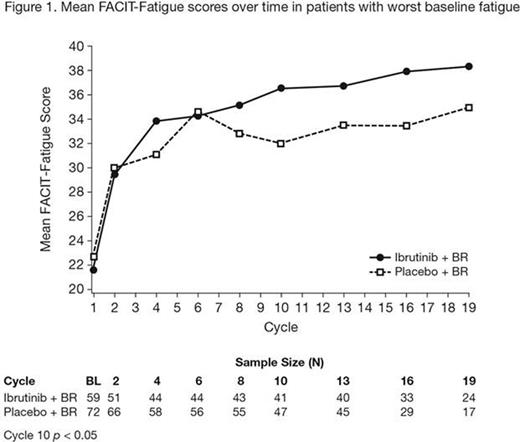
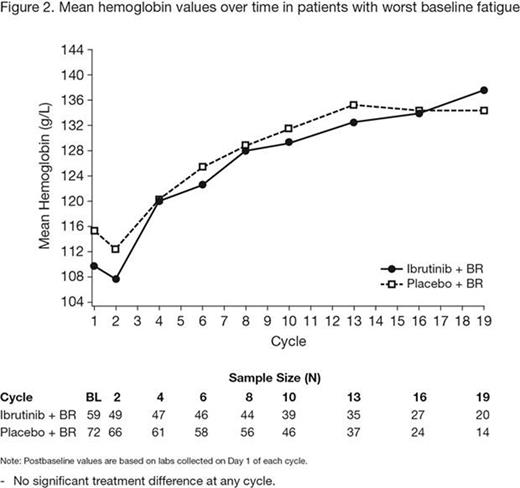
Results: Of the 578 patients enrolled, 540 (93%) provided FACIT-Fatigue responses at baseline. No notable mean changes from baseline over time were observed in any HRQL scores within or between the treatment groups. Those in the lowest fatigue score quartile (ie, worst baseline fatigue) were more likely to have an Eastern Cooperative Oncology Group performance score of 1, be in a later stage of disease, have more cytopenias, a greater number of prior therapies, and worse HRQL. Among those with the worst baseline fatigue, unadjusted results indicate apparent improvements in fatigue score over time for both treatments during the first 6 cycles of BR therapy, and subsequently greater continued improvements in fatigue were observed with ibrutinib + BR versus placebo + BR (p < 0.05 at cycle 10) (Figure 1). Similarly, for those with the worst baseline fatigue, mean hemoglobin values appear to increase over time after cycle 2, with comparable changes in each arm (Figure 2). In addition, of the patients who reported feeling ill or unwell at baseline (item 38 on CLL16), a greater proportion reported a return to wellness with ibrutinib + BR (40.0%) versus placebo + BR (27.3%) at cycle 10. This pattern was consistently observed from cycle 2 through cycle 19.
Conclusions: These data suggest that addition of ibrutinib to BR is not associated with a negative impact on HRQL and that, among patients with the worst fatigue at baseline, ibrutinib appears to be associated with greater reductions in fatigue versus placebo. Improvements in fatigue appear to be consistent with improvements in hemoglobin and improvements in CLL disease status.
Traina:Janssen: Employment. Fraser:Hoffman LaRoche: Consultancy, Honoraria; Janssen: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding. Cramer:Astellas: Other: Travel grant; Mundipharma: Other: Travel grant; Glaxo Smith Klein/Novartis: Research Funding; Janssen: Other: Travel grant, Research Funding, Speakers Bureau; Hoffman LaRoche: Other: Travel grant, Research Funding, Speakers Bureau; Gilead: Other: Travel grant, Research Funding. Santucci Silva:Novartis: Other: Travel reimbursement; Merck: Research Funding; Celgene: Research Funding; GSK: Research Funding; Janssen: Other: Travel reimbursement, Research Funding; Hoffman LaRoche: Other: Travel reimbursement, Research Funding. Dilhuydy:Roche: Honoraria, Other: Travel reimbursement; Janssen: Honoraria, Other: Travel reimbursement; Mundipharma: Honoraria. Mahler:Janssen: Employment, Other: Travel reimbursement. Salman:Janssen/J&J: Employment, Equity Ownership. Howes:Janssen/J&J: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal