Abstract
Background: The nuclear export protein XPO1 is overexpressed in all types of hematological malignancies. The SINE selinexor (KPT-330) is a novel, first-in-class, slowly reversible XPO1 antagonist that forces the nuclear retention and activation of over tumor suppressor proteins (TSPs) such as p53, IkB, FOXO and p21. Forced nuclear retention of TSPs leads to their reactivation, which can counteract a multitude of oncogenic pathways that perpetuate the neoplastic phenotype. In addition, XPO1 inhibition prevents the eIF4e-mediated export of messenger mRNA for a variety of oncoproteins (FLT3, c-KIT, cyclin D1, c-MYC, Bcl2), leading to decreased expressed to further provide anti-cancer activity.
An ongoing Phase 1 (NCT01607892) open label, dose escalation, multi-center study in hematological malignancies was designed to evaluate the safety and tolerability of selinexor as well as response rate as the secondary objective. A maximum tolerated dose (MTD) was not identified yet in this study, but based on the ongoing Phase1 study of selinexor in pts with solid tumors, a MTD of 65 mg/m2 twice weekly was determined. The goal of the analyses reported here was to identify the recommended Phase 2 dose (RP2D) based on both tolerability and efficacy in patients with heavily pretreated hematological malignancies.
Methods: A subset (N=157) of the Phase 1 patient population received oral selinexor twice weekly (8 doses/28-d cycle). General clinical observations suggested that doses of selinexor >35 mg/m2 (> ~60 mg flat dose) are associated with suboptimal tolerability. Therefore, based on the actual dose administered, patients were divided into groups receiving 45-65 mg (median 60 mg; N=59) and >65 mg (70-160 mg; median 90 mg, N=98) for comparison of safety and efficacy endpoints. The majority of the patients have heavily pretreated myeloma, NHL, and AML.
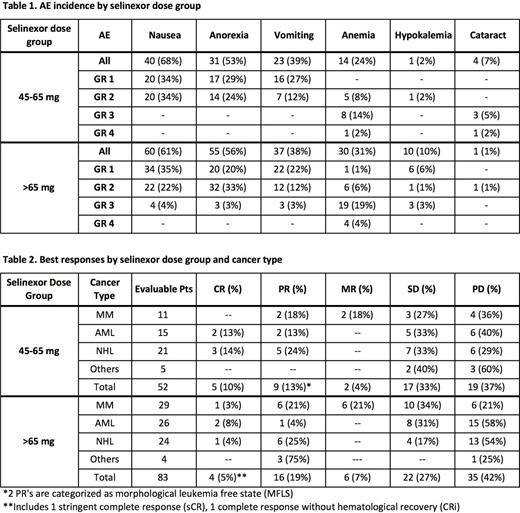
Results: 157 pts received 45-160 mg selinexor twice weekly (89 M/68 F, median age 66 yr; median 4 prior regimens). The most common adverse events (AEs) were fatigue (66%), nausea (64%), anorexia (55%), vomiting (38%), which were mostly gr 1/2, and thrombocytopenia (44%), which was mostly grade 3/4. Incidence of certain selinexor-related high grade (3/4) adverse events was greater in pts receiving >65 mg selinexor vs those receiving 45-65 mg (Table 1). Grade 3 nausea (4%), anorexia (3%), vomiting (3%) and hypokalemia (3%) were observed in the >65 mg group but were not seen in the 45-65 mg group. Grade 3 and 4 anemia were 19% vs 14% and 4% vs 2% for the >65 mg vs 45-65 mg groups, respectively. Grade 3 and 4 thrombocytopenia was similar in both groups, but slightly higher in the >65 mg group, with 8% and 24 % in the 45-65 mg group vs 12% and 27% in the >65 mg group. Neutropenia was also very similar in Grade 3 and 4 toxicity for both groups with 10% and 12% in the 45-65 mg group vs 11% and 10% in the >65 mg group. In contrast, high grade cataract was only seen in the 45-65 mg group (8%; 3 gr 3, 1 gr 4). Selinexor-induced weight loss, as compared to baseline, was maximal by the end of cycle 2 in both dose groups, without further loss through at least cycle 4, but the >65 mg group lost on average >5-fold more weight (average of 3.8 ± 1.1 kg vs 0.7 ± 0.1 kg in the 65 mg group from 56 d- 126 d; p<0.001). Also, pts in the 45-65 mg group remained on study longer (average of 101 d vs 69 d in the >65 mg group; p=0. 05). In contrast, overall efficacy in the two dose groups was comparable, with 5 complete responses (CRs, 10%) and an overall response rate (ORR) of 23% in the 45-65 mg group and 4 CRs (5%) and ORR of 24% in the >65 mg group. A listing of all responses for both groups can be seen in Table 2.
Conclusions: While efficacy is comparable, doses of selinexor from 45-65 mg (median 60 mg) are better tolerated than doses >65 mg, based upon decreased weight loss, incidence of high grade AEs, and greater numbers of days on study. Based on this superior risk-benefit, a flat dose of 60 mg selinexor, twice weekly, is the RP2D for patients with hematological cancers. Similar results have been observed for solid tumors.
Chen:Celgene: Consultancy, Honoraria, Research Funding. Jacoby:Novo Nordisk: Consultancy; Sunesis: Research Funding. Stone:AROG: Consultancy; Celator: Consultancy; Novartis: Research Funding; Pfizer: Consultancy; Juno: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; Amgen: Consultancy; Agios: Consultancy; Roche/Genetech: Consultancy; Abbvie: Consultancy; Sunesis: Consultancy, Other: DSMB for clinical trial; Merck: Consultancy. Baz:Sanofi: Research Funding; Celgene Corporation: Research Funding; Karyopharm: Research Funding; Millennium: Research Funding. Gabrail:Sanofi: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; BI: Honoraria, Speakers Bureau; Onyx: Honoraria, Speakers Bureau. Wang:Celgene: Research Funding. Martin:Janssen: Consultancy, Honoraria; Acerta: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Bayer: Consultancy. Siegel:Celgene Corporation: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Merck: Speakers Bureau. Marshall:Karyopharm: Employment. Saint-Martin:Karyopharm: Employment. Carlson:Karyopharm: Employment. Shacham:Karyopharm: Employment, Equity Ownership. Kauffman:Karyopharm: Employment, Equity Ownership. Kuruvilla:Gilead: Consultancy; Hoffmann LaRoche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Merck: Honoraria; Bristol-Myers Squibb: Honoraria; Lundbeck: Honoraria; Karyopharm: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal