Abstract
Introduction
Recombinant von Willebrand factor (rVWF, vonicog alfa, BAX 111), which is manufactured using a plasma-free method (Turecek et al Semin Thromb Hemost 2010), may provide an important alternative replacement therapy for VWD subjects. In contrast to plasma-derived (pd) VWF concentrates, BAX 111 contains a high ratio of high molecular weight (HMW) and ultralarge VWF multimers (ULM), which improve platelet and collagen binding and enhance FVIII stabilization (Kragh et al Thromb Res 2014), and may therefore be associated with better hemostatic outcomes and simplify bleeding management in VWF patients for a variety of clinical conditions. As BAX 111 is a pure VWF product, it is also not restricted to co-administration with coagulation factor VIII (FVIII). The pharmacokinetic profile of BAX 111 has been evaluated in two prospective trials (phase 1 and phase 3) in adults with severe VWD.
Methods
The pharmacokinetic profile of BAX 111 (with vs. without rFVIII; and BAX 111:rFVIII compared with a pdVWF:pdFVIII) in subjects with VWD, evaluated in a non-bleeding state, was determined in two clinical trials by assessment of ristocetin co-factor activity (VWF:RCo), VWF antigen (VWF: Ag); collagen-binding activity (VWF:CB), FVIII activity, and VWF multimer activity. Lower doses were examined in the first-in-human phase 1 dose-finding study, and the expected therapeutic doses (as recommended for licensed pdVWF-containing concentrates) of 50 IU VWF:RCo/kg and 80 IU VWF:RCo/kg (for major bleeding episodes) were selected for further evaluation in phase 3. PK assessments were also repeated after 6 months with the 80 IU VWF:RCo/kg dose.
Results
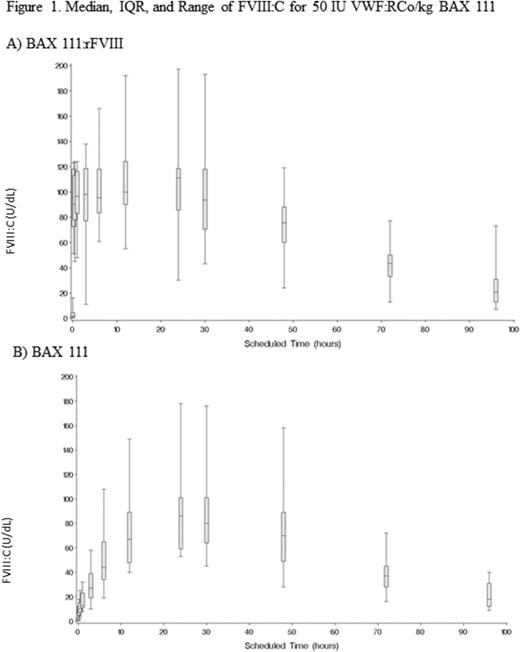
The PK profile of BAX 111 has been evaluated in a total of 56 unique subjects with severe VWD. A rapid increase in VWF:RCo was observed after a single infusion of BAX 111. There was no apparent dose-dependency at a wide range of dose levels (20 IU, 50 IU and 80 IU VWF:RCo/kg) and BAX 111 PK was not affected by administration together with rFVIII in the crossover study with the 50 IU VWF:Co/kg dose (Figure 1). A tendency toward a longer mean terminal half-life (T1/2) of VWF:RCo for BAX 111:rFVIII as compared with a pdVWF:pdFVIII was observed in the phase 1 study (23.6 h for 20 IU BAX 111:rFVIII, 19.3h for 50 IU BAX 111:rFVIII) and the mean T1/2 at 50 IU VWF:RCo/kg in the phase 3 study (19.6 h for BAX 111:rFVIII and 21.9 h for BAX 111) is also considerably longer than that of pdVWF products (Batlle et al Blood Coagul Fibrinolysis 2009). PK parameters were similar for the first (19.1 h) and second (21.2 h) assessment in the repeated PK study at 80 IU VWF:RCo/kg. VWF:CB parameters were comparable to VWF:RCo across dose levels in the phase 1 study (15.7 h for 20 IU BAX 111:rFVIII, 24.4h for 50 IU BAX 111:rFVIII) and phase 3 study (19.3h for BAX 111:rFVIII and 18.3h for BAX 111 at 50 IU; 18.8h for 80 IU in the first assessment and 20.9h 80 IU in the second assessment).
ULMs were detected after 15 minutes post-infusion of BAX 111 (earliest time point measured), followed by a substantial decline due to rapid degradation by endogenous ADAMTS13 between 12 and 24 hours.
A substantial increase in FVIII activity was observed after infusion with BAX 111 and BAX 111:rFVIII, followed by a secondary rise in FVIII activity after 12 hours due to the stabilization of endogenous FVIII, which was more pronounced for BAX 111:rFVIII than for pdVWF:pdFVIII (geometric mean ratio for AUC0-inf = 1.36 [90% Clopper-Pearson confidence interval: 0.93; 1.99]), and similar for BAX 111 alone as compared with BAX 111:rFVIII, confirming the ability of BAX 111 to stabilize endogenous FVIII:C.
Conclusion
BAX 111 has an enhanced PK profile compared to plasma-derived concentrates and there is no apparent dose dependency over a wide dose range (20 IU, 50 IU, 80 IU VWF:RCo/kg). The ULM present in BAX 111 may be more effective in stabilizing endogenous FVIII, and therefore BAX 111 may have the potential for less-frequent administration compared with plasma-derived VWF concentrates, and for FVIII-independent dosing after the initial infusion or in non-emergency bleeds.
Ragni:Biogen: Research Funding; Biomarin: Research Funding; Genentech Roche: Research Funding; Vascular Medicine Institute: Research Funding; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding; SPARK: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Research Funding; Tacere Benitec: Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; Dimension: Research Funding; CSL Behring: Research Funding; Baxalta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Castaman:Bayer, Baxalta, CSL Behring, Kedrion, Novo Nordisk, and Pfizer: Membership on an entity's Board of Directors or advisory committees. Gill:Bayer: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees. Kouides:CSL Behring: Membership on an entity's Board of Directors or advisory committees. Chapman:Baxalta: Employment. Sytkowski:Baxalta: Employment. Obermann-Slupetzky:Baxalta: Employment. Presch:Baxalta: Employment. Fritsch:Baxalta: Employment. Ewenstein:Baxalta: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal